151238
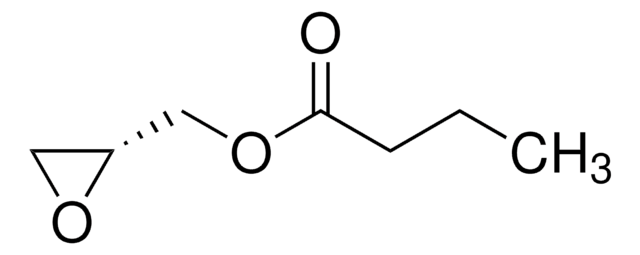
Glycidyl methacrylate
97%, contains 100 ppm monomethyl ether hydroquinone as inhibitor
동의어(들):
2,3-Epoxypropyl methacrylate, Methacrylic acid 2,3-epoxypropyl ester
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C7H10O3
CAS Number:
Molecular Weight:
142.15
Beilstein:
2506
EC Number:
MDL number:
UNSPSC 코드:
12162002
PubChem Substance ID:
NACRES:
NA.23
추천 제품
Quality Level
분석
97%
양식
liquid
포함
100 ppm monomethyl ether hydroquinone as inhibitor
불순물
0.02% epichlorohydrin
refractive index
n20/D 1.449 (lit.)
bp
189 °C (lit.)
density
1.042 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
CC(=C)C(=O)OCC1CO1
InChI
1S/C7H10O3/c1-5(2)7(8)10-4-6-3-9-6/h6H,1,3-4H2,2H3
InChI key
VOZRXNHHFUQHIL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Glycidyl methacrylate (GMA) is a polyfunctional monomer. It acts as an adhesion promoting crosslinking co-monomer for acrylic and vinyl resins. It is also a reactive colorless diluent. GMA is soluble in ethanol, acetone, diethyl ether, benzene.
애플리케이션
Glycidyl methacrylate dextran (GMA) has been reported to be used as a biocompatible hydrogel. In situ polymerization of GMA with trimethylolpropane trimethacrylate to form macroporous sorbents has also been reported. GMA may also be grafted onto polypropylene.
신호어
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Carc. 1B - Eye Dam. 1 - Muta. 2 - Repr. 1B - Skin Corr. 1C - Skin Sens. 1 - STOT RE 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
168.8 °F - closed cup
Flash Point (°C)
76 °C - closed cup
이미 열람한 고객
Styrene-assisted melt free radical grafting of glycidyl methacrylate onto polypropylene
Cartier H and Hu GH
Journal of Polymer Science Part A: Polymer Chemistry, 36(7), 1053-1063 (1998)
Molded? macroporous poly (glycidyl methacrylate-co-trimethylolpropane trimethacrylate) materials with fine controlled porous properties: preparation of monoliths using photoinitiated polymerization
Viklund C, et al
Chemistry of Materials, 9(2), 463-471 (1997)
Adam Blanazs et al.
Journal of the American Chemical Society, 133(41), 16581-16587 (2011-08-19)
Amphiphilic diblock copolymers composed of two covalently linked, chemically distinct chains can be considered to be biological mimics of cell membrane-forming lipid molecules, but with typically more than an order of magnitude increase in molecular weight. These macromolecular amphiphiles are
Synthesis, characterization, and polymerization of glycidyl methacrylate derivatized dextran.
van Dijk-Wolthuis WNE, et al.
Macromolecules, 28(18), 6317-6322 (1995)
Hye Sung Kim et al.
Biomaterials, 269, 120214-120214 (2020-08-02)
Cartilage defect is difficult to heal due to its avascular properties. Implantation of mesenchymal stem cell is one of the most promising approach for regenerating cartilage defects. Here we prepared polymeric nanofibrils decorated with cartilage-derived decellularized extracellular matrix (dECM) as
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.