모든 사진(1)
About This Item
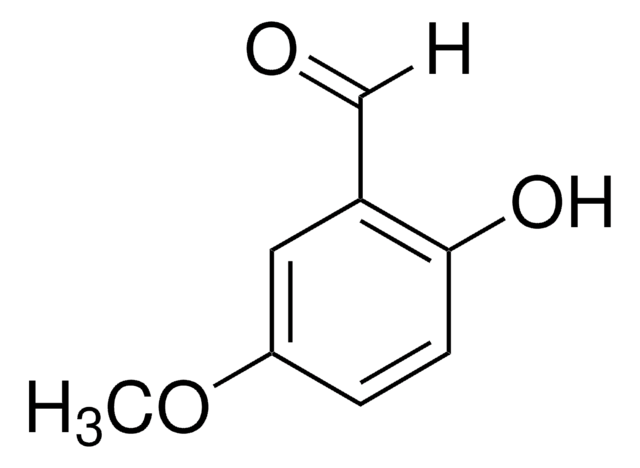
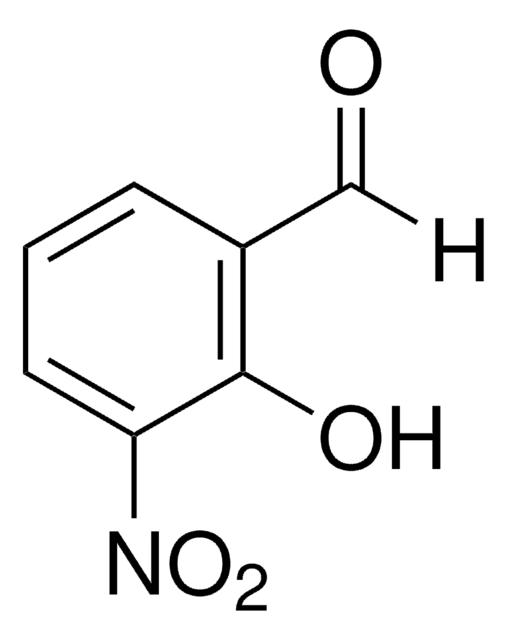
Linear Formula:
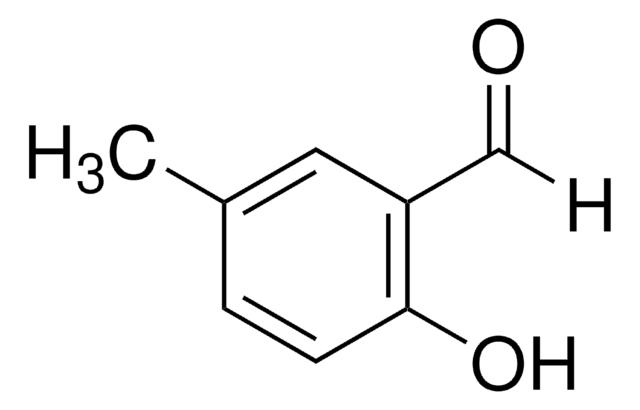
HOC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
152.15
Beilstein:
1072443
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
mp
41-43 °C (lit.)
작용기
aldehyde
SMILES string
[H]C(=O)c1ccc(OC)cc1O
InChI
1S/C8H8O3/c1-11-7-3-2-6(5-9)8(10)4-7/h2-5,10H,1H3
InChI key
WZUODJNEIXSNEU-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Hydroxy-4-methoxybenzaldehyde is the main component of root bark essential oil of Periploca sepium Bunge. It is a potential tyrosinase inhibitor present in African medicinal plants.
애플리케이션
2-Hydroxy-4-methoxybenzaldehyde was used in the synthesis of Schiff base ligand.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Dipjyoti Chakraborty et al.
Journal of plant physiology, 165(10), 1033-1040 (2007-11-21)
The fragrant rootstocks of Hemidesmus indicus are known to accumulate 2-hydroxy-4-methoxybenzaldehyde (MBALD), yet, the enzymatic route to this hydroxybenzoate is not known. Therefore, root organs of H. indicus hold promises to unravel the biosynthesis related to this phenolic fragrance. Chitosan
Gholamreza Karimipour et al.
Biological trace element research, 145(1), 109-117 (2011-08-13)
In this study, a new sorbent based on the gold nanoparticle loaded in activated carbon (Au-NP-AC) was synthesized and modified by bis(4-methoxy salicylaldehyde)-1,2-phenylenediamine (BMSAPD). This sorbent, which is abbreviated as Au-NP-AC-BMSAPD, has been applied for the enrichment and preconcentration of
P Giridhar et al.
Indian journal of experimental biology, 42(1), 106-110 (2004-07-28)
Axillary buds obtained from field grown plants of D. hamiltonii were used to initiate multiple shoots on Murashige and Skoog's medium (MS) supplemented with 2 mg L(-1) 6-benzyl aminopurine (BA) and 0.5 mg L(-1) indole-3-acetic acid (IAA). Profuse rooting was
Subban Nagarajan et al.
Journal of AOAC International, 86(3), 564-567 (2003-07-11)
The roots of Decalepis hamiltonii and Hemidesmus indicus are aromatic and possess the crystalline compound 2-hydroxy-4-methoxybenzaldehyde as the major compound (> 90%) in their volatile oils. A gas chromatographic procedure was developed for the assay of 2-hydroxy-4-methoxybenzaldehyde in both fresh
Belagihalli M Srikanta et al.
Biochimie, 93(4), 678-688 (2010-12-28)
Helicobacter pylori mediated gastric ulcer and cancers are common global problems since it was found to colonize in ∼50% of gastric ulcer/cancer patients. Decalepis hamiltonii, (Asclepiadaceae family) extracts have been depicted with medicinal properties supporting the traditional knowledge of health
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.