120804
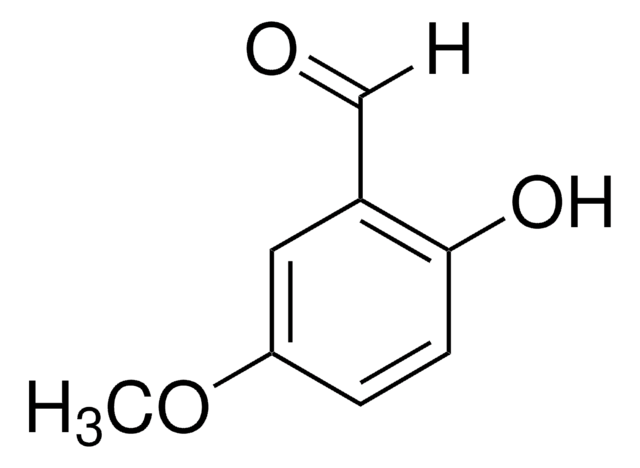
o-Vanillin
99%
동의어(들):
2-Hydroxy-3-methoxybenzaldehyde, 2-Hydroxy-m-anisaldehyde, 3-Methoxysalicylaldehyde
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
CH3OC6H3-2-(OH)CHO
CAS Number:
Molecular Weight:
152.15
Beilstein:
471913
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
양식
solid
bp
265-266 °C (lit.)
mp
40-42 °C (lit.)
작용기
aldehyde
SMILES string
COc1cccc(C=O)c1O
InChI
1S/C8H8O3/c1-11-7-4-2-3-6(5-9)8(7)10/h2-5,10H,1H3
InChI key
JJVNINGBHGBWJH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
o-Vanillin is a building block commonly used in the synthesis of schiff-base ligands.
애플리케이션
o-Vanillin has been used to study the solvent-free reaction between o-vanillin and p-toluidine using NMR, DSC and XRD analysis. It was used in the synthesis of new ligand for Fe(III) and Al(lII).
생화학적/생리학적 작용
o-Vanillin induces DNA damage as detected by comet assay.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup - (External MSDS)
Flash Point (°C)
113 °C - closed cup - (External MSDS)
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Li-Jun Ru et al.
Molecules (Basel, Switzerland), 23(7) (2018-07-14)
A self-assembled ZnII-NdIII heterohexanuclear coordination compound [Zn₄Nd₂(L)₄(bdc)₂]·2NO₃ based on a hexadentate Salamo-like chelating ligand (H₂L = 1,2-bis(3-methoxysalicylideneaminooxy)ethane]) and H₂bdc (H₂bdc = terephthalic acid) has been synthesized and characterized by elemental analyses, IR and UV/Vis spectra, and X-ray crystallography. Two crystallographically
Vanillin and o-vanillin oligomers as models for dendrimer disassembly
Robert K M et al.
New Journal of Science, 36, 492-505 (2012)
G Frenzilli et al.
Mutation research, 468(2), 93-108 (2000-07-07)
To validate the alkaline single cell gel (SCG) assay as a tool for the detection of DNA damage in human leukocytes, we investigated the in vitro activity of 18 chemicals. Thirteen of these chemicals (pyrene (PY), benzo(a)pyrene (BaP), cyclophosphamide (CP)
Y-L Ma et al.
The Journal of physiology, 590(9), 2095-2105 (2012-03-14)
The abnormally high cation permeability in red blood cells (RBCs) from patients with sickle cell disease (SCD) occupies a central role in pathogenesis. Sickle RBC properties are notably heterogeneous, however, thus limiting conventional flux techniques that necessarily average out the
K Watanabe et al.
Mutation research, 218(2), 105-109 (1989-09-01)
2-Hydroxy-3-methoxybenzaldehyde (omicron-vanillin), the antimutagenic effect of which has been reported on mutagenesis induced by 4-nitroquinoline 1-oxide (4NQO) in Escherichia coli WP2s, enhanced N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced mutagenesis in the same strain. A remarkable enhancement of mutagenesis provoked by N-methyl-N-nitrosourea (MNU) was also
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.