모든 사진(1)
About This Item
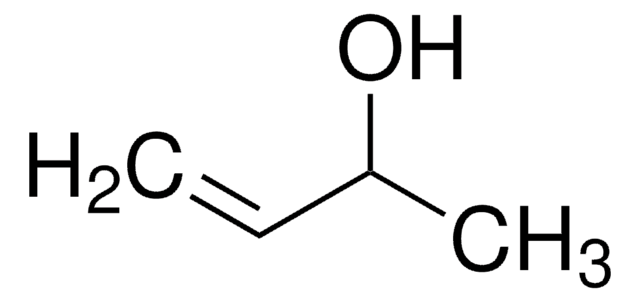
Linear Formula:
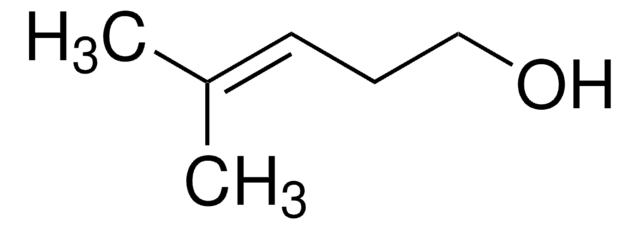
(CH3)2C=CHCH2OH
CAS Number:
Molecular Weight:
86.13
Beilstein:
1633479
EC Number:
MDL number:
UNSPSC 코드:
12352100
eCl@ss:
39020334
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor pressure
1.4 mmHg ( 20 °C)
분석
99%
양식
liquid
expl. lim.
16.3 %
refractive index
n20/D 1.443 (lit.)
bp
140 °C (lit.)
density
0.848 g/mL at 25 °C (lit.)
작용기
hydroxyl
SMILES string
C\C(C)=C\CO
InChI
1S/C5H10O/c1-5(2)3-4-6/h3,6H,4H2,1-2H3
InChI key
ASUAYTHWZCLXAN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
3-Methyl-2-buten-1-ol reacts with nitrosocarbonyl benzene to yield 5-hydroxy-isoxazolidines. It is commonly used as fragrance ingredient.
애플리케이션
3-Methyl-2-buten-1-ol was used as starting reagent during asymmetric total syntheses of (R)-(+)- and (S)-(-)-umbelactone via Sharpless asymmetric epoxidation reaction.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
124.7 °F - closed cup
Flash Point (°C)
51.5 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Anastasia Zerva et al.
Molecules (Basel, Switzerland), 23(9) (2018-09-22)
Feruloyl esterases (FAEs, E.C. 3.1.1.73) are biotechnologically important enzymes with several applications in ferulic acid production from biomass, but also in synthesis of hydroxycinnamic acid derivatives. The use of such biocatalysts in commercial processes can become feasible by their immobilization
Takeshi Bamba et al.
Journal of separation science, 32(15-16), 2699-2706 (2009-07-17)
Monolithic silica columns have very low back-pressures and offer several advantages over conventional columns packed with spherical particles, such as high separation efficiency and rapid analysis. In this review, we report the applicability of monolithic silica columns for the analysis
Branko Radetich et al.
Journal of the American Chemical Society, 124(11), 2430-2431 (2002-03-14)
A solution is reported to the classic unsolved problem of stereoselective synthesis of all-E oligoprenols, such as E-farnesylfarnesol, by a cationic coupling analogous to the biosynthetic pathway. The simplicity and efficacy of the method, which is outlined in Scheme 1
Vijay Gnanadesikan et al.
Journal of the American Chemical Society, 130(25), 8089-8093 (2008-05-23)
An effective strategy has been developed for the efficient site-selective epoxidation of poylolefinic isoprenoid alcohols, based on the use of an internal control element for intramolecular reaction. The approach is illustrated by application to a series of polyisoprenoid alcohols (polyprenols)
Lan Yang et al.
Fitoterapia, 82(6), 834-840 (2011-05-21)
The hepatoprotective effects of polyprenols from Ginkgo biloba L. leaves were evaluated against carbon tetrachloride induced hepatic damage in Sprague-Dawley rats. The elevated levels of serum ALT, AST, ALP, ALB, TP, HA, LN, TG, and CHO were restored towards normalization
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.