184225
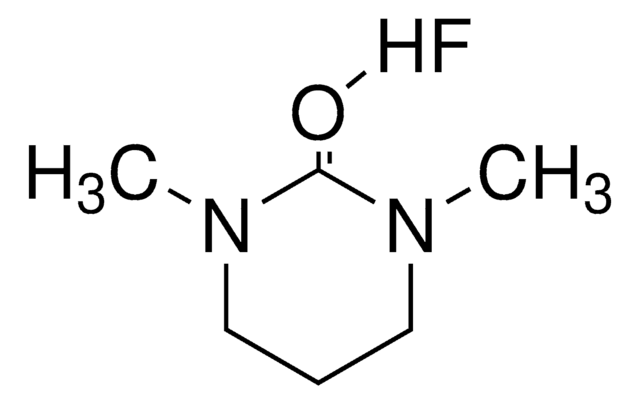
Hydrogen fluoride pyridine
pyridine ~30 %, hydrogen fluoride ~70 %
동의어(들):
HF-Pyridine, Olah′s reagent, PPHF, Poly(pyridine fluoride), Pyridine hydrofluoride, Pyridinium poly(hydrogen fluoride), Pyridinium polybifluoride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
CAS Number:
Beilstein:
4750151
MDL number:
UNSPSC 코드:
12352301
PubChem Substance ID:
NACRES:
NA.22
추천 제품
양식
liquid
Quality Level
구성
hydrogen fluoride, ~70%
pyridine, ~30%
density
1.1 g/mL at 20 °C (lit.)
저장 온도
−20°C
SMILES string
F[H].c1ccncc1
InChI
1S/C5H5N.FH/c1-2-4-6-5-3-1;/h1-5H;1H
InChI key
GRJJQCWNZGRKAU-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
May contain or form low levels of calcium fluoride during storage. Presence of this does not impact the specification values.
애플리케이션
Convenient form of anhydrous HF, stable up to 50°C. Has been used for the preparation of β-fluoroamines from amino alcohols and for the fluorination of acetylenes.
Used together with hypervalent iodine(III) reagents for ipso-fluorination of para-substituted phenols providing cyclohexadienones. Employed with Selectfluor® (Catalog No. 439479) for geminal fluorination of 2,2-diaryl-1,3-dithiolanes.
Used together with hypervalent iodine(III) reagents for ipso-fluorination of para-substituted phenols providing cyclohexadienones. Employed with Selectfluor® (Catalog No. 439479) for geminal fluorination of 2,2-diaryl-1,3-dithiolanes.
Reactant for preparation of:
Reagent for:
- Epimers of shikimic acid with the features of fucosylated glycans via zinc-mediated reductive ring opening followed by a Barbier reaction
- Vaccinia H1-related (VHR) phosphatase inhibitor with a nonacidic phosphate-mimicking core structure
- Candidates for nucleic acid drugs
- ω-substituted gem-difluoroalkanes by oxidative desulfurization-difluorination
Reagent for:
- Halofluorination reactions
법적 정보
Selectfluor is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Journal of Fluorine Chemistry, 37, 343-343 (1987)
Tetrahedron, 60, 6629-6629 (2004)
Tetrahedron Letters, 32, 69-69 (1991)
V Prakash Reddy et al.
Chemical communications (Cambridge, England), (5), 654-656 (2005-01-27)
2,2-diaryl-1,3-dithiolanes, readily obtainable from diaryl ketones, were transformed into the corresponding gem-difluoro compounds using a novel reagent combination involving Selectfluor and pyridinium polyhydrogen fluoride (PPHF) under mild conditions in moderate to good yields.
Hui Shen et al.
Infection and immunity, 83(7), 2694-2704 (2015-04-22)
Fungi can shield surface pathogen-associated molecular patterns (PAMPs) for evading host immune attack. The most common and opportunistic human pathogen, Candida albicans, can shield β-(1 3)-glucan on the cell wall, one of the major PAMPs, to avoid host phagocyte Dectin-1
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.



![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)