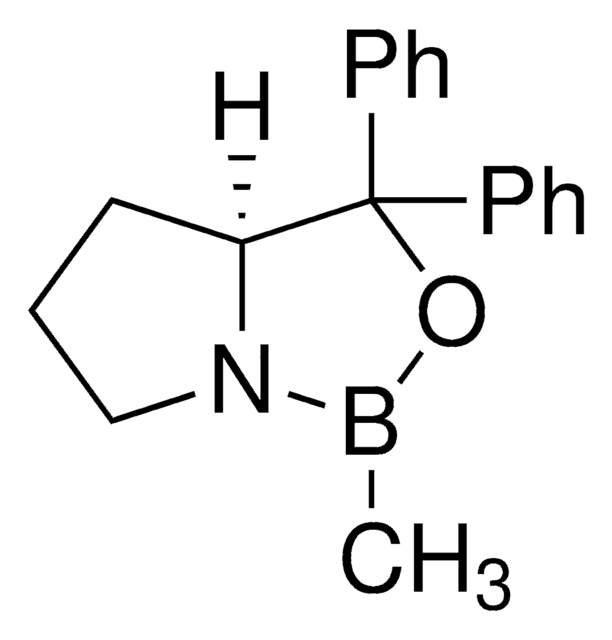
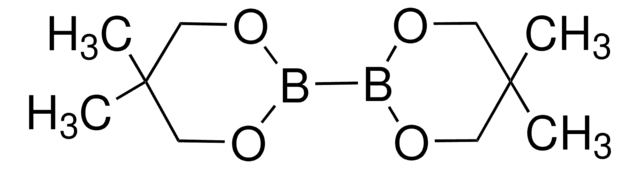
추천 제품
Quality Level
분석
98%
양식
liquid
반응 적합성
reagent type: reductant
refractive index
n20/D 1.507 (lit.)
bp
50 °C/50 mmHg (lit.)
mp
12 °C (lit.)
density
1.125 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
[bH]1oc2ccccc2o1
InChI
1S/C6H5BO2/c1-2-4-6-5(3-1)8-7-9-6/h1-4,7H
InChI key
CENMEJUYOOMFFZ-UHFFFAOYSA-N
애플리케이션
A monofunctional hydroborating agent which reduces β-hydroxyketones to 1,3-diols. Effects conjugate reduction of α,β-enones.
Used to prepare B-alkylcatecholboranes which were used, in turn, to generate alkyl radicals forming aryl ethers from quinones. Employed in a preparation of C2-symmetric boron complexes from methylenebis(oxazolines) used for enantioselective reduction of ketones.
법적 정보
Made under U.S. Pat. No. 6,204,405.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
35.6 °F - closed cup
Flash Point (°C)
2 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
The Journal of Organic Chemistry, 55, 5678-5678 (1990)
Eveline Kumli et al.
Organic letters, 8(25), 5861-5864 (2006-12-01)
Addition of alkyl radicals generated from B-alkylcatecholboranes onto 1,4-benzoquinones leads to substituted hydroquinones in good overall yields. Formation of aryl ethers via a unique radical addition to the oxygen atom of the enone system is the main reaction when bulky
The Journal of Organic Chemistry, 55, 5190-5190 (1990)
European Journal of Organic Chemistry, 4596-4596 (2006)
문서
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)

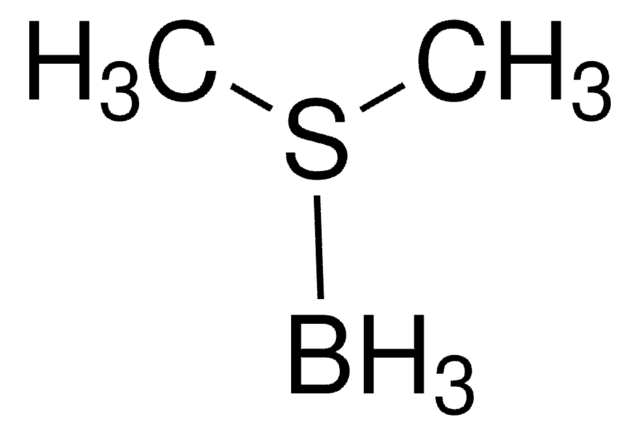




![9-Borabicyclo[3.3.1]nonane dimer](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)