모든 사진(2)
About This Item
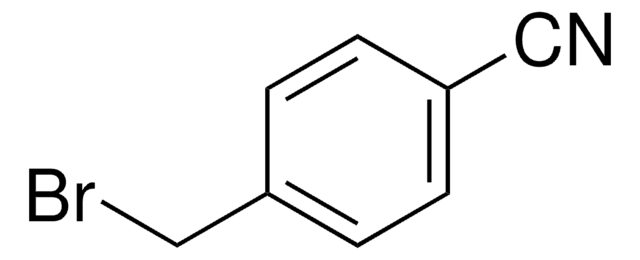
Linear Formula:
[(CH3)2CHCH2]2AlH
CAS Number:
Molecular Weight:
142.22
Beilstein:
4123663
MDL number:
UNSPSC 코드:
12352001
eCl@ss:
38120609
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Diisobutylaluminum hydride solution (1M in toluene) has been used for the reduction of aziridine esters to the corresponding aldehydes. It has also been used in the synthesis of dialkylaluminum alkoxides from alcohols.
Used in Pd-catalyzed reductive debromination of secondary alkyl bromides. O-Debenzylation and ring opening of perbenzylated furanosides. Convenient in situ generation of HZrCp2Cl from ZrCp2Cl2 and DIBAL-H.
포장
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
법적 정보
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
신호어
Danger
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT RE 2 Inhalation - STOT SE 3 - Water-react 1
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point (°F)
39.2 °F - closed cup
Flash Point (°C)
4 °C - closed cup
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Macromolecular engineering of polyactones and polyactides. 12. Study of the depolymerization reactions of poly (. epsilon.-caprolactone) with functional aluminum alkoxide end groups.
Dubois P, et al.
Macromolecules, 26(17), 4407-4412 (1993)
Synthesis of peptide macrocycles using unprotected amino aldehydes.
Rotstein BH, et al.
Nature Protocols, 5(11), 1813-1813 (2010)
Hidetsura Cho et al.
The Journal of organic chemistry, 75(3), 627-636 (2009-12-31)
A systematic investigation of the reductive ring-expansion reaction of cyclic ketoximes fused to aromatic rings with diisobutylaluminum hydride (DIBALH) is described. This reaction regioselectively afforded a variety of five- to eight-membered bicyclic heterocycles or tricyclic heterocycles containing nitrogen neighboring an
D J Kopecky et al.
The Journal of organic chemistry, 65(1), 191-198 (2000-05-18)
An optimized protocol for the DIBALH reductive acetylation of acyclic esters and diesters is described. This reductive acetylation procedure allows a wide variety of esters to be converted into the corresponding alpha-acetoxy ethers in good to excellent yields. It was
Y Kitade et al.
Nucleic acids symposium series, (27)(27), 107-108 (1992-01-01)
Reaction of purine nucleosides, such as 2',3'-isopropylideneinosine (1a) and 2',3'-isopropylideneadenosine (1c), with diisobutylaluminum hydride (DIBAL) in dry tetrahydrofurane resulted in the formation of the corresponding 9-(2',3'-isopropylideneribity)purines (2) in good yields. Oxidation of the ribityl derivatives (2) with NalO4 and subsequent
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.