추천 제품
Quality Level
분석
98%
양식
powder
반응 적합성
reaction type: solution phase peptide synthesis
mp
134-136 °C (lit.)
응용 분야
peptide synthesis
SMILES string
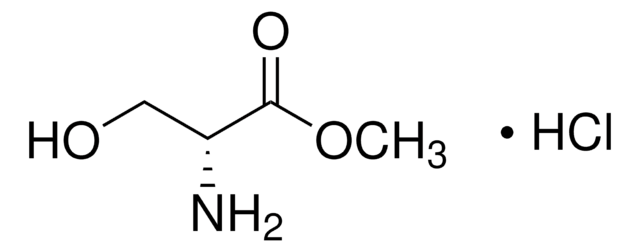
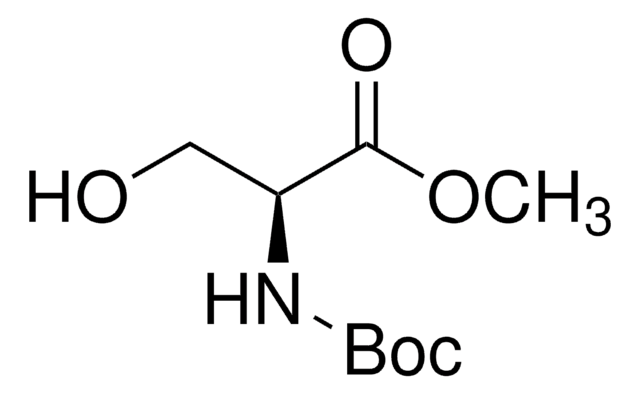
Cl[H].COC(=O)C(N)CO
InChI
1S/C4H9NO3.ClH/c1-8-4(7)3(5)2-6;/h3,6H,2,5H2,1H3;1H
InChI key
NDBQJIBNNUJNHA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Carlos Aydillo et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(17), 4840-4848 (2007-03-17)
A new chiral serine equivalent and its enantiomer have been synthesized from (S)- and (R)-N-Boc-serine methyl esters (Boc: tert-butyloxycarbonyl). The use of these compounds as chiral building blocks has been demonstrated in the synthesis of alpha-alkyl alpha-amino acids by diastereoselective
Yu Harayama et al.
Chemical communications (Cambridge, England), (13)(13), 1764-1766 (2005-03-26)
The use of hypervalent iodine(III) reagents allowed us to develop the novel and efficient direct synthesis of N,O-acetal compounds via the oxidative fragmentation reaction of alpha-amino acids or alpha-amino alcohols; furthermore, we succeeded in developing an improved synthesis of the
J Sélambarom et al.
Carbohydrate research, 330(1), 43-51 (2001-02-24)
The reaction of L-serine methyl ester hydrochloride (1) with paraformaldehyde (2) in dichloromethane in the presence of triethylamine afforded a novel compound: [lS,2S,6S,7S]-1,6-diaza-4,9-dioxa-2,7-dimethoxycarbonylbicyclo[4.4.1]undecane (4) as a 2:3 adduct of 1 with 2. 1H and 13C NMR spectroscopy were unable to
Cédric Couturier et al.
Organic letters, 8(10), 2183-2186 (2006-05-05)
[reaction: see text] Reaction of N,N-dibenzyl-O-methylsulfonyl serine methyl ester with a variety of heteronucleophiles (sodium azide, sodium phthalimide, amines, thiols) and carbanions (sodium malonate) gave, via an aziridinium intermediate, the corresponding beta-amino or alpha,beta-diamino ester in good to excellent yield.
Dragana Ahel et al.
FEBS letters, 579(20), 4344-4348 (2005-08-02)
Seryl-tRNA synthetases (SerRSs) fall into two distinct evolutionary groups of enzymes, bacterial and methanogenic. These two types of SerRSs display only minimal sequence similarity, primarily within the class II conserved motifs, and possess distinct modes of tRNA(Ser) recognition. In order
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.