추천 제품
vapor pressure
760 mmHg ( 78 °C)
Quality Level
분석
95%
형태
liquid
refractive index
n20/D 1.376 (lit.)
bp
78 °C (lit.)
solubility
alcohol: miscible(lit.)
diethyl ether: miscible(lit.)
density
0.882 g/mL at 25 °C (lit.)
작용기
O-nitroso
nitroso
저장 온도
2-8°C
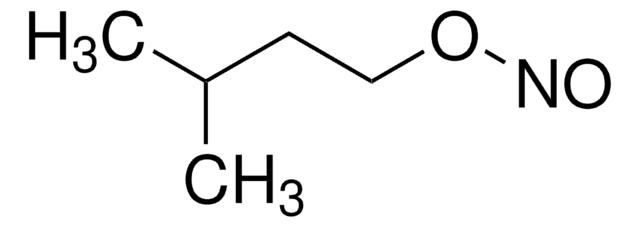
SMILES string
CCCCON=O
InChI
1S/C4H9NO2/c1-2-3-4-7-5-6/h2-4H2,1H3
InChI key
JQJPBYFTQAANLE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The photodissociation dynamics of butyl nitrite was studied using time-resolved Fourier transform infrared (TR-FTIR) emission spectroscopy. The effects of butyl nitrite on methyl cobalamin and 5-methyl tetrahydrofolate were studied.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
8.6 °F - closed cup
Flash Point (°C)
-13 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Min Ji et al.
The Journal of chemical physics, 130(17), 174314-174314 (2009-05-12)
We report on the photodissociation dynamics study of n-butyl nitrite and isoamyl nitrite by means of time-resolved Fourier transform infrared (TR-FTIR) emission spectroscopy. The obtained TR-FTIR emission spectra of the nascent NO fragments produced in the 355 nm laser photolysis
B A Meloche et al.
Xenobiotica; the fate of foreign compounds in biological systems, 23(8), 863-871 (1993-08-01)
1. The addition of n-butyl nitrite (BN) to isolated rat hepatocytes caused rapid S-nitrosyl glutathione (GSNO) formation, then a concomitant decrease in protein thiols, followed by a marked ATP depletion. Cytotoxic concentrations of BN also caused lipid peroxidation after a
G R Newell et al.
Pharmacotherapy, 4(5), 284-291 (1984-09-01)
Volatile nitrite in the form of amyl nitrite was used for 100 years for the treatment of angina pectoris. In spite of recognized toxicity, its use in this form was considered safe. During the 1960s prescriptions were not required for
G R Newell et al.
The American journal of medicine, 78(5), 811-816 (1985-05-01)
Early reports of the acquired immune deficiency syndrome (AIDS) in homosexual men suggested that the cause might be related to homosexual life-style practices, including use of recreational drugs. Inhalation of volatile nitrites is a possible contributing factor in AIDS because
J D Osterloh et al.
Journal of pharmaceutical sciences, 74(7), 780-782 (1985-07-01)
The uptake of butyl nitrite by rats (500 g, one rat/chamber) was determined over a 5-min exposure period. About 44% of the starting amount (771-3855 ppm) of n-butyl nitrite was consumed in 5 min. Three rats per exposure concentration were
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.