추천 제품
Grade
for synthesis
Quality Level
vapor density
4.9 (vs air)
vapor pressure
4.2 mmHg ( 20 °C)
형태
liquid
expl. lim.
36 %
반응 적합성
core: boron
reagent type: Lewis acid
reagent type: catalyst
refractive index
n20/D 1.344 (lit.)
bp
126-129 °C (lit.)
mp
−58 °C (lit.)
density
1.15 g/mL (lit.)
작용기
ether
저장 온도
2-8°C
SMILES string
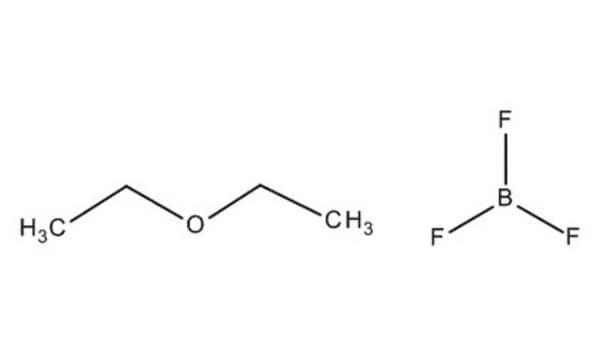
CC[O+](CC)[B-](F)(F)F
InChI
1S/C4H10BF3O/c1-3-9(4-2)5(6,7)8/h3-4H2,1-2H3
InChI key
MZTVMRUDEFSYGQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Boron trifluoride diethyl etherate or boron trifluoride-ether complex is an organic compound widely used as a convenient source of boron trifluoride (BF3) in organic synthesis. It is also used as a Lewis acid for the activation of electrophiles.
애플리케이션
Catalyst used in the preparation of cyclopentyl- and cycloheptyl[b]indoles from aryl cyclopropyl ketones via [3+2] cycloaddition.
Lewis acid reagent with broad application
포장
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
법적 정보
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT RE 1 Inhalation
표적 기관
Kidney
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
137.3 °F - closed cup
Flash Point (°C)
58.5 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
European Journal of Organic Chemistry, 5378-5378 (2006)
M S F Lie Ken Jie et al.
Chemistry and physics of lipids, 125(2), 93-101 (2003-09-23)
Methyl santalbate (methyl trans-11-octadecen-9-ynoate) from Sandal wood seed oil, Santalbum alum) was epoxidized to methyl trans-11,12-epoxy-octadec-9-ynoate (1). Treatment of compound 1 with tetrabutylammonium dihydrogentrifluoride, and boron trifluoride etherate gave the corresponding anti- (2a) (57%) and syn- (2b) (35%) fluorohydrin derivatives
George R Pettit et al.
Journal of natural products, 74(9), 1922-1930 (2011-09-09)
The synthesis of bis-steroidal pyrazines derived from 3-oxo-11,21-dihydroxypregna-4,17(20)-diene (4) and glycosylation of a D-ring side chain with α-L-rhamnose have been summarized. Rearrangement of steroidal pyrazine 10 to 14 was found to occur with boron triflouride etherate. Glycosylation of pyrazine 10
Hui Xu et al.
Bioorganic & medicinal chemistry letters, 21(13), 4008-4012 (2011-06-03)
Twenty-one 4α-acyloxy-2-chloropodophyllotoxin derivatives (5a-u), whose C-4 spatial configuration was mainly stereocontrolled by the configuration of C-2 chlorine atom, were unexpectedly prepared by the reaction of 2-chloropodophyllotoxin with carboxylic acids in the presence of BF(3)·Et(2)O. Compared with ordinary esterifications of carboxylic
U C Reddy et al.
The Journal of organic chemistry, 74(6), 2605-2608 (2009-02-17)
A diastereoselective one-pot, three-component Prins-Friedel-Crafts reaction was developed for the synthesis of 4-aryltetrahydropyran derivatives from the reaction of carbonyl compounds with homoallylic alcohol in the presence of arene promoted by boron trifluoride etherate.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.