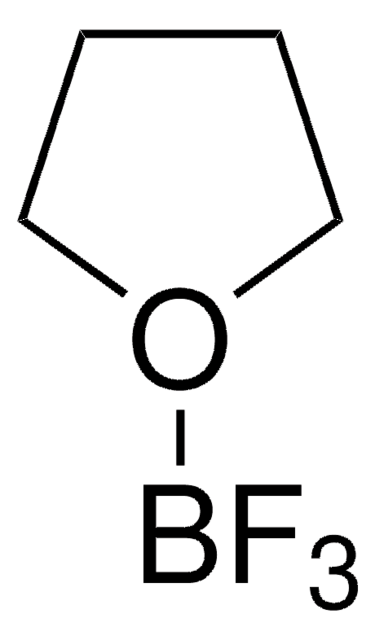
176192
Borane tetrahydrofuran complex solution
1.0 M in THF
동의어(들):
Borane-THF (1:1), Boron hydride (BH3)-THF (1:1), Tetrahydrofuran-trihydroborane complex, Trihydrido(tetrahydrofuran)boron
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
BH3OC4H8
CAS Number:
Molecular Weight:
85.94
Beilstein:
3668402
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
포함
<0.005 M sodium borohydride as stabilizer
Quality Level
반응 적합성
reagent type: reductant
농도
1.0 M in THF
density
0.898 g/mL at 25 °C
작용기
ether
저장 온도
2-8°C
SMILES string
B.C1CCOC1
InChI
1S/C4H8O.BH3/c1-2-4-5-3-1;/h1-4H2;1H3
InChI key
RMCYTHFAWCWRFA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Borane tetrahydrofuran complex (BH3-THF) is widely used as a reducing agent in organic synthesis. It is also used as a reagent in hydroboration reactions.
애플리케이션
BH3-THF can be used as a reducing agent for the reduction of various functional groups such as carboxylic acids, aldehydes, ketones, esters, acid chlorides, nitriles, epoxides, amides, lactones, oximes, and imines into corresponding alcohols and amines. Grignard reagents, arylmercury, arylthalium, and allyl and propargyllithium compounds react with BH3−THF to give organoboranes, which can be oxidized to the corresponding alcohols, phenols, and 1,3-diols.
It can also be used:
It can also be used:
- To synthesize the chiral borane catalyst, which is used in the enantioselective halo-aldol reaction.
- To prepare 9-unsubstituted acridines by reduction of corresponding acridones.
- To reduce nylon surface amide groups to secondary amines.
포장
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
법적 정보
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - STOT SE 3 - Water-react 1
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
Simple and convenient conversion of acridones into 9-unsubstituted acridines via acridanes using borane tetrahydrofuran complex
Desbois N, et al.
Tetrahedron Letters, 50(49), 6894-6896 (2009)
Borane-Tetrahydrofuran
Zaidlewicz M, et al.
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Nylon surface modification. Part 1. Targeting the amide groups for selective introduction of reactive functionalities
Jia X, et al.
Polymer, 47(14), 4916-4924 (2006)
Polymer, 47, 4916-4916 (2006)
Jung Seok Lee et al.
Macromolecular bioscience, 15(9), 1314-1322 (2015-06-04)
We designed poly(β-amino esters) (PBAEs) bearing both UV light- and pH-sensitive groups and used PBAEs to prepare nanoparticles (NPs) that can be utilized for on-demand burst release of guest molecules in response to multiple triggers. Due to the presence of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)