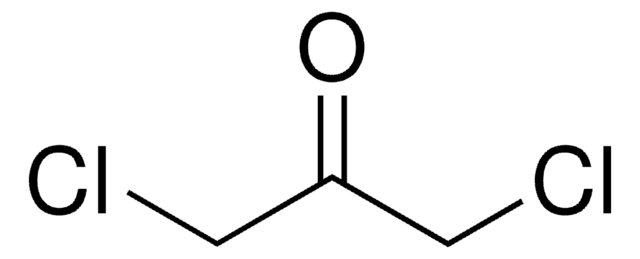
추천 제품
형태
liquid
Quality Level
반응 적합성
reagent type: reductant
농도
2.0 M in THF
density
0.855 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
B.CSC
InChI
1S/C2H6S.BH3/c1-3-2;/h1-2H3;1H3
InChI key
RMHDLBZYPISZOI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Borane dimethyl sulfide complex (BMS) acts as a highly efficient and selective reducing agent in the presence of catalytic sodium tetrahydroborate for α-hydroxy esters. Asymmetric borane reduction of a variety of prochiral ketones with BMS using spiroborate esters as catalyst has been reported.
애플리케이션
Borane dimethyl sulfide complex (BMS) solution may be used in the following studies:
- One-pot conversion of alkynes into 1,2-diols.
- Preparation of iminopentitols.
- Reduction of representative organic functional groups, such as esters, nitriles and amides.
- Used along with a dendrimeric supported L-pyrrolidinol in the asymmetric reduction of indanones and tetralones.
- Asymmetric reduction of ferrocenyl-1,3-diketones to chiral 1,3-diols.
- Highly enantioselective reduction of ketones catalyzed by C3-symmetric tripodal hydroxyamides.
Reactant used as a regioselective reducing agent
Reactant involved in:
Reactant involved in:
- Hydroboration / oxidation
신호어
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 1B - STOT SE 3 - Water-react 1
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Viatcheslav Stepanenko et al.
Tetrahedron letters, 48(33), 5799-5802 (2007-08-13)
Novel spiroborate esters derived nonracemic 1,2-aminoalcohols and ethylene glycol are reported as highly effective catalysts for the asymmetric borane reduction of a variety of prochiral ketones with borane-dimethyl sulfide complex at room temperature. Optically active alcohols were obtained in excellent
Ana M Gómez et al.
Organic letters, 4(3), 383-386 (2002-02-01)
Primary ozonides derived from alkenyl stannanes display an unusual stability and can be transformed into 1,2-diols by treatment with dimethyl sulfide and borane-methyl sulfide complex. This observation has been incorporated into the development of a novel one-pot strategy for the
M Godskesen et al.
Bioorganic & medicinal chemistry, 4(11), 1857-1865 (1996-11-01)
The four stereoisomeric 1,5-dideoxy-1,5-iminopentitols with D-arabino-(D-lyxo-) (3), ribo- (9), L-lyxo (L-arabino-) (13) and xylo-(18) configurations were synthesized. The corresponding aldonolactones (1, 7 and 11) or aldonic acid ester (15b) having a leaving group at C-5 gave by reaction with aqueous
Selective reductions. 29. A simple technique to achieve an enhanced rate of reduction of representative organic compounds by borane-dimethyl sulfide.
Brown HC, et al.
The Journal of Organic Chemistry, 47(16), 3153-3163 (1982)
Highly Enantioselective Borane Reduction of Prochiral Ketones Catalyzed by C3-Symmetric Tripodal β-Hydroxy Amides
Fang T, et al.
Synlett, 1559-1559 (2006)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.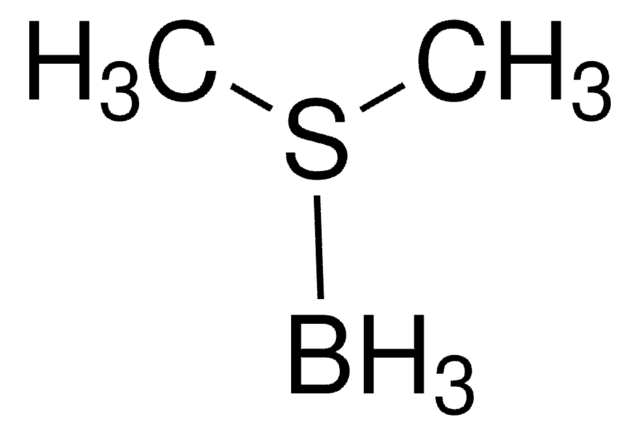



![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)