모든 사진(1)
About This Item
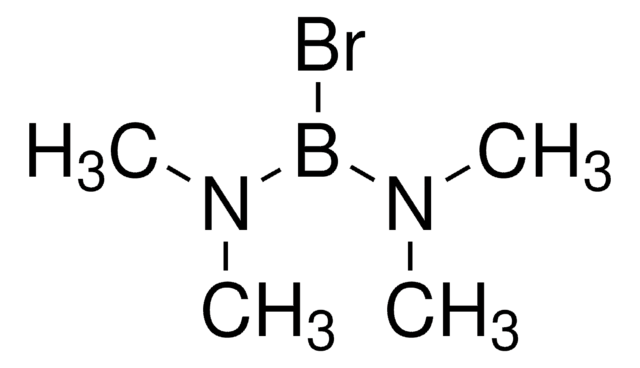
실험식(Hill 표기법):
BBr3
CAS Number:
Molecular Weight:
250.52
EC Number:
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Boron tribromide (BBr3) is a strong Lewis acid generally used as a reagent for the deprotection of ethers. Alkyl aryl ethers are cleaved at the alkyl-oxygen bond to give ArOH and alkyl bromides. In a particular case, BBr3 was used to cleave acetals that could not be deprotected under standard acidic conditions. Similarly, amino acid-protecting groups such as benzyloxycarbonyl and tert-butoxycarbonyl groups can be deprotected by BBr3. It can also be used to deprotect carbohydrate derivatives and polyoxygenated intermediates in the preparation of deoxyvernolepin, vernolepin, and vernomenin.
애플리케이션
Reactant for preparation of:
- Drug intermediate 6-nitro-L-DOPA
- Luminescent polystyrene derivatives with sterically protected carbazolylborane moieties
- High-quality boron-doped graphene via Wurtz-type reductive coupling reaction
- Mercapto-(+)-methamphetamine haptens for synthesis of (+)-methamphetamine conjugate vaccines with improved epitope densities
- Micrometer-sized organic molecule-DNA hybrid structures
- Borane complexes via electrophilic aromatic borylation reactions
- A 5-HT2C receptor agonist
- Biphenyl-derivatives possessing tertiary amino groups as β-secretase(BACE1) inhibitors for the treatment of Alzheimer′s disease
- A highly near-IR region fluorescent p-extended boron aza-dipyrromethene moiety unit
- Tetrahydroisoquinoline derivatives via intramolecular cyclization of methoxy-substituted N-phenethylimides
With alkynes forms 2-alkenyldibromoboranes, which show reversed regiochemistry in Diels-Alder reactions as compared to BBN. Intermediates generated from 1-alkynes couple to alkyl halides providing trisubstituted alkenes. Reacts with chiral sulfonamides to provide precursors of chiral glycidol esters, acetate diols, β-hydroxy esters, and amino acid esters. Useful for the synthesis of precursors to group 13-15 semiconductor materials.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Boron trichloride/tetra-n-butylammonium iodide: A mild, selective combination reagent for the cleavage of primary alkyl aryl ethers
Brooks PR, et al.
The Journal of Organic Chemistry, 64(26), 9719-9721 (1999)
Tetrahedron Letters, 29, 1811-1811 (1988)
Michael S. Lube et al.
Inorganic chemistry, 35(17), 5007-5014 (1996-08-14)
The 1:1 mole ratio reactions of boron trihalides (BX(3)) with tris(trimethylsilyl)phosphine [P(SiMe(3))(3)] produced 1:1 Lewis acid/base adducts [X(3)B.P(SiMe(3))(3), X = Cl (1), Br (2), I (5)]. Analogous 1:1 mole ratio reactions of these boron trihalides with lithium bis(trimethylsilyl)phosphide [LiP(SiMe(3))(2)] produced
Tetrahedron Letters, 34, 3071-3071 (1993)
Ether Cleavage Re-Investigated: Elucidating the Mechanism of BBr3-Facilitated Demethylation of Aryl Methyl Ethers
Kosak TM, et al.
European Journal of Organic Chemistry, 2015(34), 7460-7467 (2015)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.