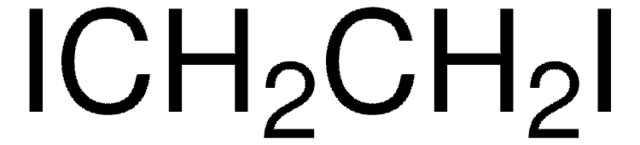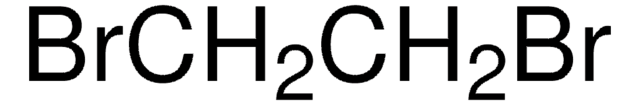추천 제품
vapor density
6.8 (vs air)
Quality Level
vapor pressure
2 mmHg ( 20 °C)
분석
99%
양식
liquid
포함
copper as stabilizer
refractive index
n20/D 1.548 (lit.)
bp
170-172 °C (lit.)
density
1.904 g/mL at 25 °C (lit.)
작용기
chloro
iodo
SMILES string
ClCCCI
InChI
1S/C3H6ClI/c4-2-1-3-5/h1-3H2
InChI key
SFOYQZYQTQDRIY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1-Chloro-3-iodopropane undergoes asymmetric α-alkylation with N-sulfinyl imidates to yield 2-substituted N-tert-butanesulfinyl-5-chloropentanimidates. Electroreduction of 1-chloro-3-iodopropane at glassy carbon electrode in dimethylformamide containing tetra-n-butylammonium perchlorate has been investigated by cyclic voltammetry. It also participates in conjugate addition of alkyl iodides to α,β-unsaturated nitriles in water.
애플리케이션
1-Chloro-3-iodopropane has been used in the synthesis of:
- N-[4-[5-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5-yl)-2-(2,2,2-trifluoroacetyl)pentyl]benzoyl]-L-glutamic acid, an inhibitor of glycinamide ribonucleotide transformylase (GAR Tfase) and aminoimidazole carboxamide ribonucleotide transformylase (AICAR Tfase)
- interesting ″proton sponge″ type molecule quino[7,8-h]quinoline
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Synthesis of 1, 2, 3, 4-tetrahydroquinolines and 1, 2, 3, 4-tetrahydro-1, 6-naphthyridines by a directed lithiation reaction.
Reed JN, et al.
Tetrahedron Letters, 29(45), 5725-5728 (1988)
Electrochemical Reduction of 1, 3-Dihalopropanes at Carbon Cathodes in Dimethylformamide.
Pritts WA and Peters DG.
Journal of the Electrochemical Society, 141(4), 990-995 (1994)
Fraser F Fleming et al.
The Journal of organic chemistry, 72(18), 6961-6969 (2007-08-10)
A new silica-supported zinc-copper matrix reagent promotes the conjugate addition of alkyl iodides to cyclic and acyclic alkenenitriles in water. X-ray diffraction and electron microscopy techniques suggest that the active copper species generated from elemental zinc and copper(I) iodide is
Filip Colpaert et al.
The Journal of organic chemistry, 76(1), 234-244 (2010-12-02)
α-Alkylation of N-sulfinyl imidates with 1-chloro-3-iodopropane successfully led to 2-substituted N-tert-butanesulfinyl-5-chloropentanimidates in acceptable diastereomeric ratios (dr 67/33 to 72/28) and good yields (74-86%). Subsequent reduction with NaBH(4) led to the corresponding 2-substituted N-tert-butanesulfinyl-5-chloropentylamines, which could be cyclized to a range
Heng Cheng et al.
Bioorganic & medicinal chemistry, 13(10), 3593-3599 (2005-04-26)
The synthesis and evaluation of N-[4-[5-(2,4-diamino-6-oxo-1,6-dihydropyrimidin-5-yl)-2-(2,2,2-trifluoroacetyl)pentyl]benzoyl]-L-glutamic acid (2) as an inhibitor of glycinamide ribonucleotide transformylase (GAR Tfase) and aminoimidazole carboxamide ribonucleotide transformylase (AICAR Tfase) are reported. The inhibitor 2 was prepared in a convergent synthesis involving C-alkylation of methyl 4-(4,4,4-trifluoro-3-dimethylhydrazonobutyl)benzoate
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.