252794
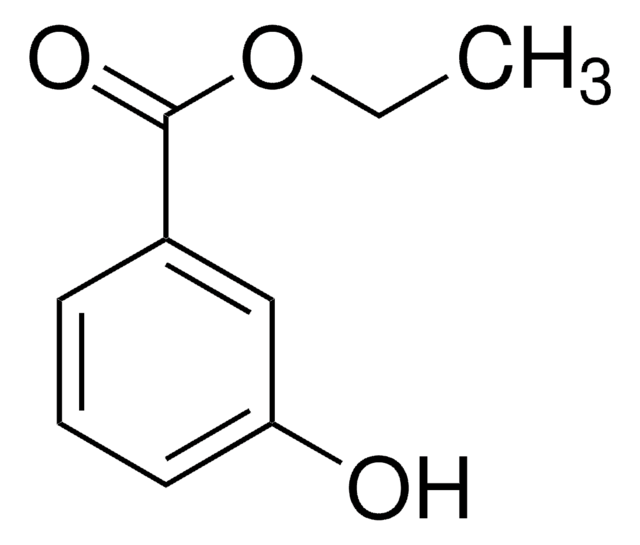
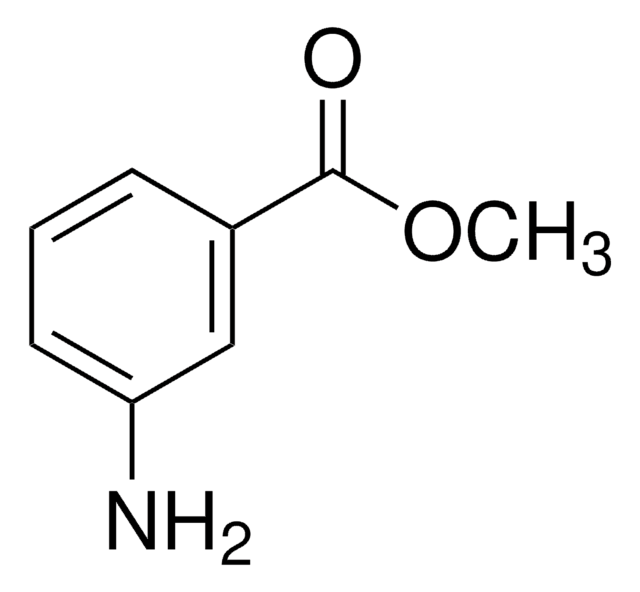
Methyl 3-hydroxybenzoate
99%
동의어(들):
3-(Methoxycarbonyl)phenol, 3-Carbomethoxyphenol, 3-Hydroxybenzoic acid methyl ester, Methyl m-hydroxybenzoate, m-Carbomethoxyphenol, m-Hydroxybenzoic acid methyl ester
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
HOC6H4CO2CH3
CAS Number:
Molecular Weight:
152.15
Beilstein:
2208129
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Methyl 3-hydroxybenzoate has been used in synthesis of:
- 3-hydroxybenzene-1,2-dicarbaldehyde
- O-methyl O-[3-methyl-4-(methylthio)phenyl]O-(3-methylcarboxyphenyl) phosphorothioate
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Investigation of the effect of hapten heterology on immunoassay sensitivity and development of an enzyme-linked immunosorbent assay for the organophosphorus insecticide fenthion.
Kim YJ, et al.
Analytica Chimica Acta, 94(1, 29-40 (2003)
T Sakata et al.
Bioscience, biotechnology, and biochemistry, 65(10), 2315-2317 (2002-01-05)
The structure of a novel aromatic compound contained in the unidentified Rhizoglyhus mite (Acaridae: Astigmata) was elucidated, without its isolation, to be 3-hydroxybenzene-1,2-dicarbaldehyde (tentatively named gamma-acaridial) by a combination of GC/MS and GC/FT-IR together with knowledge of related mite compounds.
Deze Kong et al.
Science advances, 6(44) (2020-11-01)
Chalcone synthase (CHS) canonically catalyzes carbon-carbon bond formation through iterative decarboxylative Claisen condensation. Here, we characterize a previously unidentified biosynthetic capability of SlCHS to catalyze nitrogen-carbon bond formation, leading to the production of a hydroxycinnamic acid amide (HCAA) compound. By
Shota Machida et al.
Molecules (Basel, Switzerland), 24(23) (2019-12-01)
Twenty-one natural and unnatural phenolic compounds containing a carbohydrate moiety were synthesized and their structure-activity relationship (SAR) was evaluated for α-glucosidase inhibition and antioxidative activity. Varying the position of the galloyl unit on the 1,5-anhydro-d-glucitol (1,5-AG) core resulted in changes
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.