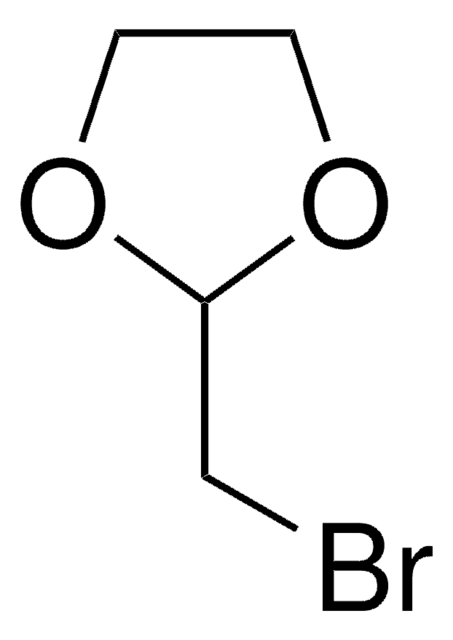
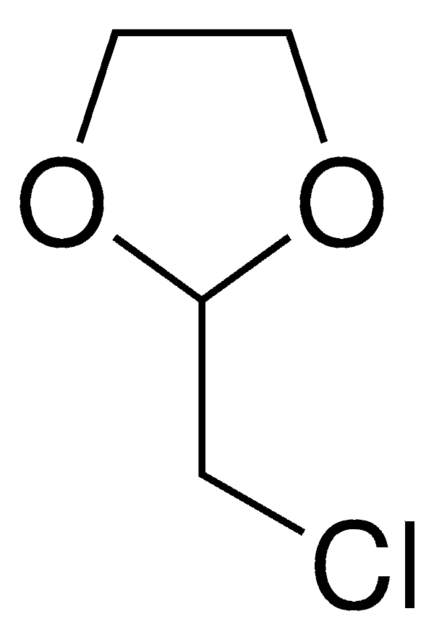
추천 제품
Quality Level
분석
≥97.0% (GC)
형태
liquid
refractive index
n20/D 1.453
bp
93-94 °C/12 mmHg (lit.)
density
1.142 g/mL at 20 °C (lit.)
SMILES string
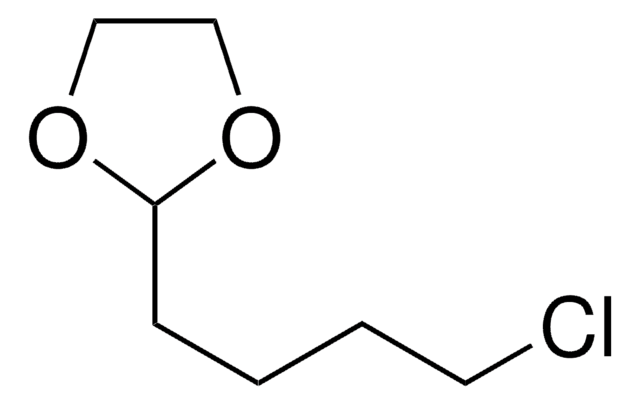
ClCCCC1OCCO1
InChI
1S/C6H11ClO2/c7-3-1-2-6-8-4-5-9-6/h6H,1-5H2
InChI key
ZBPUNVFDQXYNDY-UHFFFAOYSA-N
애플리케이션
2-(3-Chloropropyl)-1,3-dioxolane (2-(3′-chloropropyl)-1,3-dioxolane) is a masked γ-chlorobutyraldehyde and was used for the introduction of 3-(1,3-dioxolan-2-yl)propyl moiety. It was also used in the synthesis of:
- (±)-histrionicotoxin and (±)-histrionicotoxin 235A using a two-directional strategy
- 4-iodobutyraldehyde, 5-iodovaleraldehyde and 5-iodo-2-petanone
- corresponding phosphonate
기타 정보
Masked γ-chlorobutyraldehyde, useful for the introduction of the 3-(1,3-dioxolan-2-yl)propyl moiety; Preparation and use of the corresponding phosphonate
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
174.2 °F - closed cup
Flash Point (°C)
79 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
C.P. Forbes et al.
Journal of the Chemical Society. Perkin Transactions 1, 2353-2353 (1977)
R.E. Abbott et al.
The Journal of Organic Chemistry, 45, 5398-5398 (1980)
S.A. Bal et al.
The Journal of Organic Chemistry, 47, 5045-5045 (1982)
A Nagy et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2464-2469 (1996-03-19)
A convenient, high yield conversion of doxorubicin to 3'-deamino-3'-(2''-pyrroline-1''-yl)doxorubicin is described. This daunosamine-modified analog of doxorubicin is 500-1000 times more active in vitro than doxorubicin. The conversion is effected by using a 30-fold excess of 4-iodobutyraldehyde in anhydrous dimethylformamide. The
P.A. Aristoff
The Journal of Organic Chemistry, 50, 1765-1765 (1985)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.