29590
(1,5-Cyclooctadiene)(pyridine)(tricyclohexylphosphine)-iridium(I) hexafluorophosphate
≥99.0% (C)
동의어(들):
[Ir(cod)(PCy3)(py)]PF6, (1,5-Cyclooctadiene)(pyridine)(tricyclohexylphosphine)-Ir(I) PF6, Crabtree’s catalyst, Iridium(I) hexafluorophosphate (1,5-Cyclooctadiene)-(pyridine)-(tricyclohexylphosphine) complex, [Ir(cod)(PCy3)(py)]PF6
About This Item
추천 제품
Quality Level
분석
≥99.0% (C)
반응 적합성
core: iridium
reagent type: catalyst
mp
175 °C (dec.) (lit.)
SMILES string
[Ir+].c1ccncc1.F[P-](F)(F)(F)(F)F.C2CC=CCCC=C2.C3CCC(CC3)P(C4CCCCC4)C5CCCCC5
InChI
1S/C18H33P.C8H12.C5H5N.F6P.Ir/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-2-4-6-8-7-5-3-1;1-2-4-6-5-3-1;1-7(2,3,4,5)6;/h16-18H,1-15H2;1-2,7-8H,3-6H2;1-5H;;/q;;;-1;+1/b;2-1-,8-7-;;;
InChI key
UJXHUUQZACSUOG-KJWGIZLLSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
포장
기타 정보
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
관련 콘텐츠
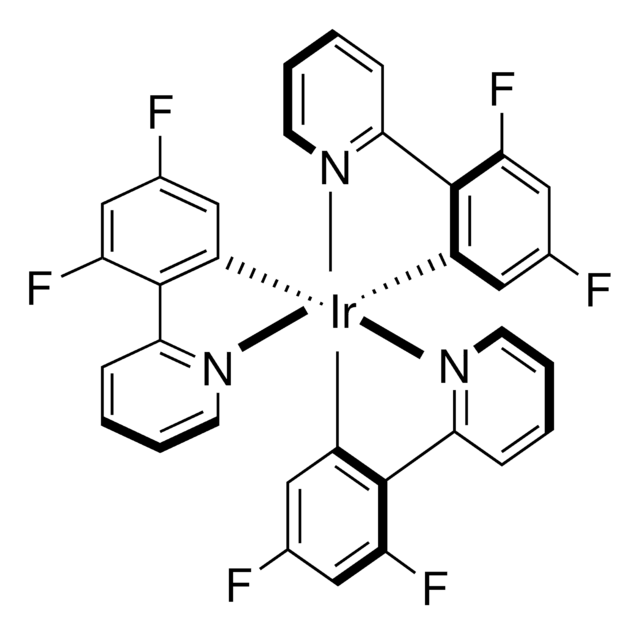
The Crabtree Group developed the [(cod)IrLL']PF6 series of catalysts, where L is a P-donor such as PCy3 and L' and N-donor such as pyridine. These are very active in the hydrogenation of sterically hindered alkenes. The catalyst also shows directing effects as a result of binding of the catalyst to suitable functional groups on the substrate, followed by addition of H2 from the same side of the substrate that the functional group is located. Two versions of the catalyst are available from us one with PF6 and the other with BArF4 counteranion.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![[Ir(cod)(acac)] Umicore](/deepweb/assets/sigmaaldrich/product/structures/188/615/470bfca9-6b61-476a-9486-f7da61962e4c/640/470bfca9-6b61-476a-9486-f7da61962e4c.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)




![1,5-Cyclooctadiene{[dibenzyl((4S,5S)-5-methyl-2-phenyl-4,5-dihydro-4-oxazolyl)methyl]dicyclohexylphosphinite κN:κP}iridium(I) tetrakis(3,5-bis(trifluoromethyl)phenyl)borate 97%](/deepweb/assets/sigmaaldrich/product/structures/139/575/e2052bbf-fcaa-4d37-a53a-3cab3894162b/640/e2052bbf-fcaa-4d37-a53a-3cab3894162b.png)
![1,5-Cyclooctadiene{[dibenzyl((4R,5R)-5-methyl-2-phenyl-4,5-dihydro-4-oxazolyl)methyl]dicyclohexylphosphinite κN:κP}iridium(I) tetrakis(3,5-bis(trifluoromethyl)phenyl)borate 97%](/deepweb/assets/sigmaaldrich/product/structures/109/838/8e9b273f-4ee4-4ff5-a3f7-cebe84a877bc/640/8e9b273f-4ee4-4ff5-a3f7-cebe84a877bc.png)