추천 제품
Grade
technical grade
Quality Level
양식
powder
mp
290-292 °C (lit.)
SMILES string
O=C1Nc2ccccc2-c3ccccc13
InChI
1S/C13H9NO/c15-13-11-7-2-1-5-9(11)10-6-3-4-8-12(10)14-13/h1-8H,(H,14,15)
InChI key
RZFVLEJOHSLEFR-UHFFFAOYSA-N
유전자 정보
human ... PARP1(142)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
6-(5H)-Phenanthridinone is an inhibitor of poly(ADP-ribose)polymerase (PARP)-1 activity. The ability of 6-(5H)-phenanthridinone to potentiate the effect of ionizing radiation on tumour cells was evaluated. Action of 6-(5H)-phenanthridinone, one of the most potent PARP inhibitor, on RDM4 murine lymphoma cells in culture was evaluated.
애플리케이션
Reactant involved in:
Reactant involved in the synthesis and/or pharmacological activity of biologically active molecules including:
- Synthesis of 5,6-dihydrophenanthridine sulfonamides
- Oxidative coupling with diphenylacetylene
- Direct copper acetate-catalyzed N-cyclopropylation of cyclic amides
Reactant involved in the synthesis and/or pharmacological activity of biologically active molecules including:
- Potassium channel KV1.3 and IK-1 inhibitors
- HIV-1 integrase inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Prakash Jagtap et al.
Critical care medicine, 30(5), 1071-1082 (2002-05-15)
To synthesize novel inhibitors of the nuclear enzyme poly(adenosine 5'-diphosphate [ADP]-ribose) synthetase (PARS), also known as poly(ADP-ribose) polymerase (PARP), and to test them in in vitro models of oxidant-induced cytotoxicity and in endotoxin and splanchnic occlusion-reperfusion-induced shock. Randomized, prospective laboratory
Miriam León Paumen et al.
Environmental science & technology, 42(9), 3434-3440 (2008-06-05)
This study aimed to monitor PAC availability to the oligochaete Lumbriculus variegatus during 28 days of exposure to spiked sediments, in order to obtain reliable chronic effect concentrations for reproduction. Sediment toxicity tests were performed using three pairs of PAC
Sajjad Ahmad et al.
Organic & biomolecular chemistry, 10(19), 3937-3945 (2012-04-06)
A new synthetic approach has been developed for the preparation of 7-deoxypancratistatin analogues bearing a syn-(4aS,10bS)-phenanthridone ring junction. A one-pot tandem process involving a substrate-directed Overman rearrangement and ring closing metathesis reaction was developed for the stereoselective synthesis of a
Sara Ebrahimi Nasrabady et al.
Cellular and molecular neurobiology, 31(4), 503-508 (2011-02-19)
Excitotoxicity is considered to be a major pathophysiological mechanism responsible for extensive neuronal death after acute spinal injury. The chief effector of such a neuronal death is thought to be the hyperactivation of intracellular PARP-1 that leads to cell energy
F Moroni et al.
Cell death and differentiation, 8(9), 921-932 (2001-08-30)
An excessive activation of poly(ADP-ribose) polymerase (PARP) has been proposed to play a key role in post-ischemic neuronal death. We examined the neuroprotective effects of the PARP inhibitors benzamide, 6(5H)-phenanthridinone, and 3,4-dihydro-5-[4-1(1-piperidinyl)buthoxy]-1(2H)-isoquinolinone in three rodent models of cerebral ischemia. Increasing
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.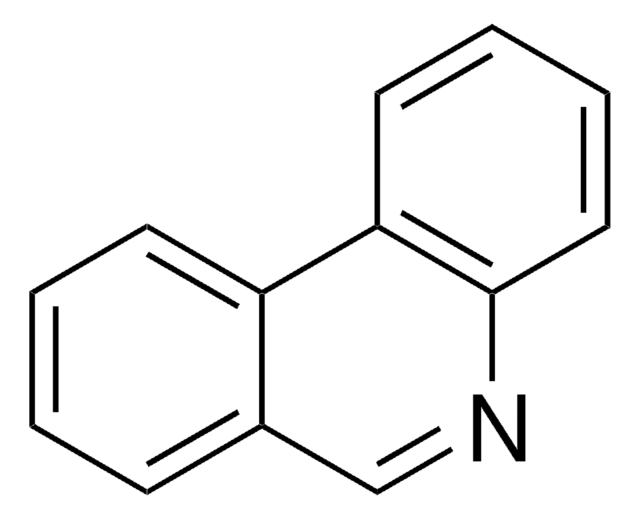

![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)