303232
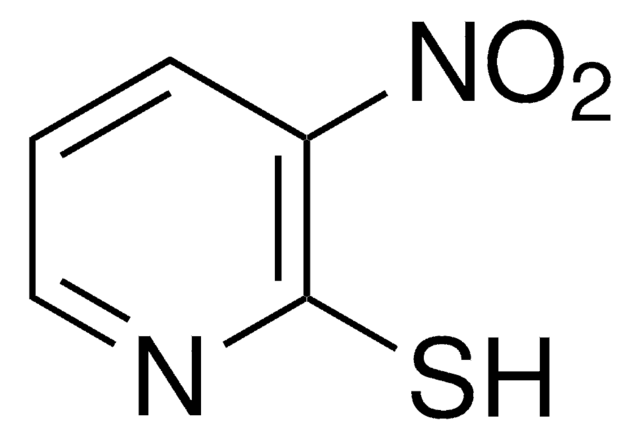
3-Nitro-2-pyridinesulfenyl chloride
95%
동의어(들):
(3-Nitro-2-pyridyl)sulfenyl chloride, 2-(Chlorosulfanyl)-3-nitropyridine, 3-Nitropyridinyl-2-sulfenyl chloride, [(3-Nitropyridin-2-yl)sulfanyl]chlorane
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C5H3ClN2O2S
CAS Number:
Molecular Weight:
190.61
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
mp
205 °C (dec.) (lit.)
solubility
dichloromethane: soluble(lit.)
작용기
nitro
저장 온도
2-8°C
SMILES string
[O-][N+](=O)c1cccnc1SCl
InChI
1S/C5H3ClN2O2S/c6-11-5-4(8(9)10)2-1-3-7-5/h1-3H
InChI key
WTKQMHWYSBWUBE-UHFFFAOYSA-N
일반 설명
The 3-nitro-2-pyridinesulphenyl (Npys) moiety is useful as a protecting-activating group for cysteine, particularly in the synthesis of cyclic and unsymmetrical disulfides. The stability of NpysCl was studied in various solvents.
애플리케이션
3-Nitro-2-pyridinesulfenyl chloride (NpysCl) was employed as the starting material for the synthesis of N-, O- and S-Npys-protected amino acid.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
R Matsueda et al.
Peptide research, 5(5), 262-264 (1992-09-01)
Two recent reports on the partial lability of the 3-nitro-2-pyridinesulfenyl (Npys) thiol protecting group towards 1-hydroxy-benzotriazole (HOBt) have prompted a rechecking of the chemical behavior of this group. Using both soluble and polymer-bound forms of Cys(Npys) as test materials, the
O Rosen et al.
International journal of peptide and protein research, 35(6), 545-549 (1990-06-01)
The hydroxylic side-chain functional groups of serine, threonine, hydroxproline and tyrosine, the alpha and epsilon-amino moieties of lysine and the thiol group of cysteine were masked by the 3-nitro-2-pyridinesulfenyl (Npys) protecting group. Deprotection was mildly affected by thiolysis with either
R G Simmonds et al.
International journal of peptide and protein research, 43(4), 363-366 (1994-04-01)
The 3-nitro-2-pyridinesulphenyl (Npys) moiety is finding increasing utility as a protecting-activating group for cysteine, particularly in the synthesis of cyclic and unsymmetrical disulfides using the Boc strategy. This chemistry has been extended to peptides assembled by the Fmoc strategy. N-Terminal
S Rajagopalan et al.
International journal of peptide and protein research, 45(2), 173-179 (1995-02-01)
TASPs (template-assembled synthetic peptides) are generated by the covalent attachment of linear peptides to a common peptide backbone, thus generating larger synthetic peptides/proteins with prefolded structure. In this work we present a strategy for the synthesis of a heterotemplate-assembled synthetic
R Matsueda et al.
Peptide research, 7(1), 32-35 (1994-01-01)
Thrombin-induced platelet aggregation has been suggested to play an important role in reocclusion following thrombolytic therapy or angioplasty for treatment of myocardial infarction. We previously demonstrated that thrombin-induced platelet aggregation is indirectly mediated by intracellularly activated calpain expressed on the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.