추천 제품
Grade
reagent grade
Quality Level
vapor density
5.2 (vs air)
vapor pressure
8 mmHg ( 25 °C)
제품 라인
ReagentPlus®
분석
≥99%
양식
liquid
refractive index
n20/D 1.327 (lit.)
bp
162 °C (lit.)
mp
- 40 °C
solubility
1,600 g/L at 20 °C
density
1.696 g/mL at 25 °C (lit.)
작용기
fluoro
triflate
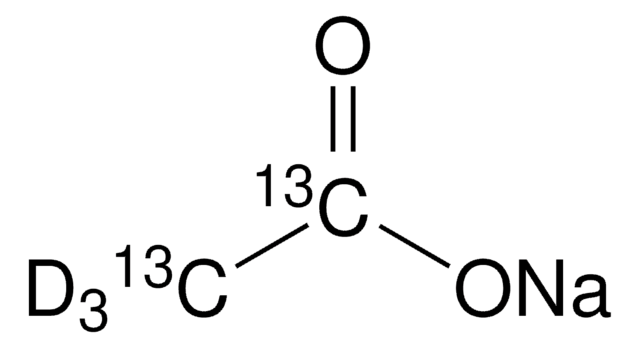
SMILES string
OS(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S/c2-1(3,4)8(5,6)7/h(H,5,6,7)
InChI key
ITMCEJHCFYSIIV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Trifluoromethanesulfonic acid is a strong organic acid. It can be prepared by reacting bis(trifluoromethylthio)mercury with H2O2. On mixing with HNO3, it affords a nitrating reagent (a nitronium salt). This reagent is useful for the nitration of aromatic compounds. Its dissociation in various organic solvents has been studied.
애플리케이션
Deglycosylation agent
Trifluoromethanesulfonic acid can be used as a catalyst to prepare:
It can also be used as a catalyst in the Fischer glycosylation and Friedel-Crafts acylation reactions.
- Substituted tetrahydrofurans and tetrahydropyrans by cyclization of corresponding unsaturated alcohols under acidic conditions.
- Nitriles from corresponding aldehydes by Schmidt reaction.
- Disubstituted five-membered ring lactones by allylboration reaction between 2-alkoxycarbonyl allylboronates and aldehydes.
It can also be used as a catalyst in the Fischer glycosylation and Friedel-Crafts acylation reactions.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Met. Corr. 1 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point (°F)
>332.1 °F - Pensky-Martens closed cup
Flash Point (°C)
> 166.7 °C - Pensky-Martens closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Use of trifluoromethanesulfonic acid in fischer glycosylations.
Wessel HP.
Journal of Carbohydrate Chemistry, 7(1), 263-269 (1988)
Chemoselective Schmidt reaction mediated by triflic acid: selective synthesis of nitriles from aldehydes
Rokade BV and Prabhu KR
The Journal of Organic Chemistry, 77(12), 5364-5370 (2012)
Triflic acid-catalysed cyclisation of unsaturated alcohols
Coulombel L and Dunach E
Green Chemistry, 6(10), 499-501 (2004)
Triflic acid-catalyzed additions of 2-alkoxycarbonyl allylboronates to aldehydes. Study of scope and mechanistic investigation of the reaction stereochemistry
Elford TG, et al.
The Journal of Organic Chemistry, 72(4), 1276-1284 (2007)
Paul D Veith et al.
mBio, 11(5) (2020-09-03)
Porphyromonas gingivalis and Tannerella forsythia use the type IX secretion system to secrete cargo proteins to the cell surface where they are anchored via glycolipids. In P. gingivalis, the glycolipid is anionic lipopolysaccharide (A-LPS), of partially known structure. Modified cargo
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.