361615
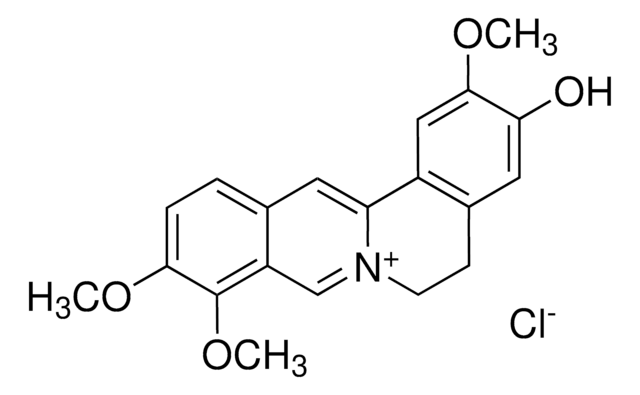
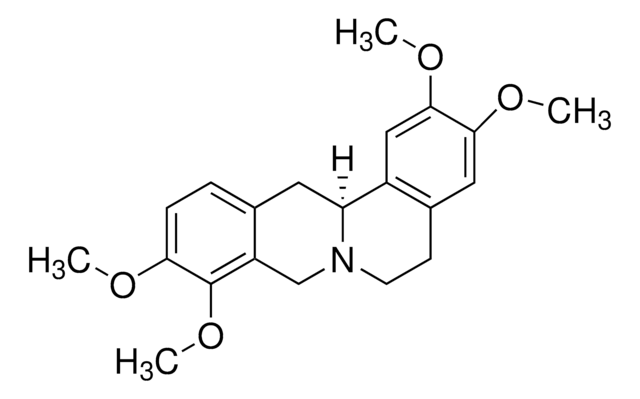
Palmatine chloride hydrate
97%
동의어(들):
5,6-Dihydro-2,3,9,10-tetramethoxydibenzo[a,g]quinolizinium chloride hydrate (1:1:1)
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C21H22ClNO4 · xH2O
CAS Number:
Molecular Weight:
387.86 (anhydrous basis)
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
불순물
~1.5 mol/mol methanol
mp
206-207 °C (dec.) (lit.)
SMILES string
[Cl-].[H]O[H].COc1cc2CC[n+]3cc4c(OC)c(OC)ccc4cc3-c2cc1OC
InChI
1S/C21H22NO4.ClH.H2O/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4;;/h5-6,9-12H,7-8H2,1-4H3;1H;1H2/q+1;;/p-1
InChI key
PIQNSCSNSSZUIT-UHFFFAOYSA-M
일반 설명
Palmatine chloride hydrate (Palmatine) is an alkaloid. It is a potential phototoxin, and exhibits low quantum yields for fluorescence. Normal Raman spectra and DFT calculations of palmatine chloride hydrate is reported.
애플리케이션
Palmatine chloride hydrate (Palmatine) is suitable for use:
- as alkaloid standard in the method validation for determination of berberine, hydrastine and canadine in goldenseal (Hydrastis canadensis L.) root powder
- in the preparation of 8-heteroaryl-7,8-dihydroprotoberberine
- in a study to investigate the FT-Raman and surface-enhanced Raman scattering (SERS) spectra of three related alkaloid dyes, namely palmatine, jatrorrhizine and coptisine
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Surface-enhanced Raman scattering of protoberberine alkaloids.
Canamares MV, et al.
Journal of Raman Spectroscopy, 39(12), 1907-1914 (2008)
Sayaka Shinji et al.
Bioscience, biotechnology, and biochemistry, 84(1), 63-75 (2019-08-30)
A natural isoquinoline alkaloid, berberine, has been known to exhibit anti-tumor activity in various cancer cells via inducing cell cycle arrest. However, it has not been investigated whether berberine and its analogs inhibit the growth of rhabdomyosarcoma (RMS), which is
Holly A Weber et al.
Journal of AOAC International, 86(3), 476-483 (2003-07-11)
A fast, practical ambient extraction methodology followed by isocratic liquid chromatography (LC) analysis with UV detection was validated for the determination of berberine, hydrastine, and canadine in goldenseal (Hydrastis canadensis L.) root powder. The method was also validated for palmatine
Sayaka Shinji et al.
Frontiers in cell and developmental biology, 8, 616706-616706 (2021-02-16)
Herein we report that the 18-base telomeric oligodeoxynucleotides (ODNs) designed from the Lactobacillus rhamnosus GG genome promote differentiation of skeletal muscle myoblasts which are myogenic precursor cells. We termed these myogenetic ODNs (myoDNs). The activity of one of the myoDNs
Lenka Grycová et al.
Magnetic resonance in chemistry : MRC, 46(12), 1127-1134 (2008-09-11)
Adducts of the quaternary protoberberine alkaloids (QPA) berberine, palmatine, and coptisine were prepared with nucleophiles derived from pyrrole, pyrazole, imidazole, and 1,2,4-triazole. The products, 8-substituted 7,8-dihydroprotoberberines, were identified by mass spectrometry and 1D and 2D NMR spectroscopy, including (1)H--(15)N shift
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.