추천 제품
Quality Level
분석
98%
bp
261 °C (lit.)
mp
40-42 °C (lit.)
작용기
bromo
nitro
저장 온도
2-8°C
SMILES string
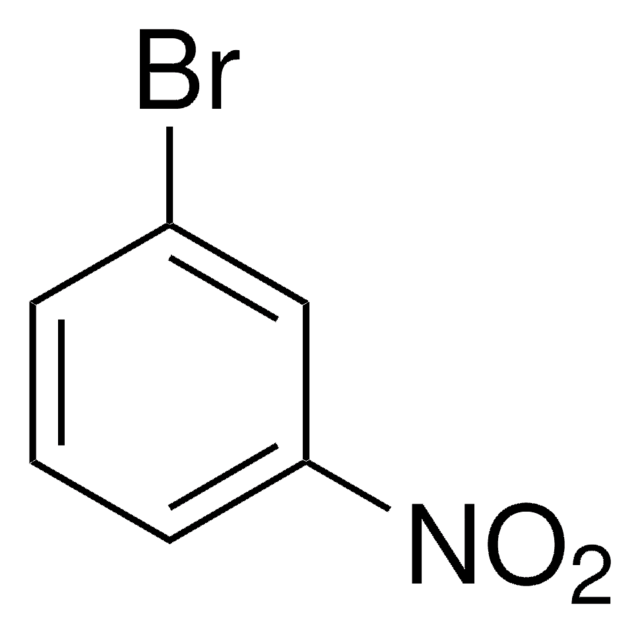
[O-][N+](=O)c1ccccc1Br
InChI
1S/C6H4BrNO2/c7-5-3-1-2-4-6(5)8(9)10/h1-4H
InChI key
ORPVVAKYSXQCJI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1-Bromo-2-nitrobenzene undergoes palladium[0]-mediated Ullmann cross-coupling reaction with a range of β-halo-enals, -enones or -esters to afford the corresponding β-aryl derivatives. Palladium[0]-mediated Ullmann cross-coupling reaction of 1-bromo-2-nitrobenzene with β-bromo-α,β-unsaturated aldehydes is reported.
애플리케이션
1-Bromo-2-nitrobenzene may be used in the preparation of:
- 4-methoxy-2′-nitrodiphenyl ether
- 1-methoxy-3,5-bis-(2-nitro-phenoxy)benzene
- 5-hydroxy-3-methoxy-2′-nitrodiphenyl ether
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
233.6 °F - closed cup
Flash Point (°C)
112 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Martin G Banwell et al.
Organic letters, 6(16), 2741-2744 (2004-07-30)
Palladium[0]-mediated Ullmann cross-coupling of 1-bromo-2-nitrobenzene (1 R = H) and its derivatives with a range of beta-halo-enals, -enones, or -esters readily affords the corresponding beta-aryl derivatives, which are converted into the corresponding quinolines, 2-quinolones, phenanthridines, or 6(5H)-phenanthridinones on reaction with
New protocols for the synthesis of 3, 4-annulated and 4-substituted quinolines from ?-bromo-a, ?-unsaturated aldehydes and 1-bromo-2-nitrobenzene or 2-bromoacetanilide.
Some S, et al.
Tetrahedron Letters, 48(20), 3609-3612 (2007)
4-Hydroxy-2'-Nitrodiphenyl Ether Analogues as Novel Tyrosinase Inhibitors.
Sapkota K, et al.
Bull. Korean Chem. Soc., 31(5), 1319-1319 (2010)
Luka A Wright et al.
Dalton transactions (Cambridge, England : 2003), 44(16), 7230-7241 (2015-03-20)
The 2-(2′-aniline)-6-imine-pyridines, 2-(C6H4-2′-NH2)-6-(CMe=NAr)C5H3N (Ar = 4-i-PrC6H4 (HL1a), 2,6-i-Pr2C6H3 (HL1b)), have been synthesised via sequential Stille cross-coupling, deprotection and condensation steps from 6-tributylstannyl-2-(2-methyl-1,3-dioxolan-2-yl)pyridine and 2-bromonitrobenzene. The palladium(II) acetate N,N,N-pincer complexes, [{2-(C6H4-2′-NH)-6-(CMe=NAr)C5H3N}Pd(OAc)] (Ar = 4-i-PrC6H4 (1a), 2,6-i-Pr2C6H3 (1b)), can be prepared by
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.