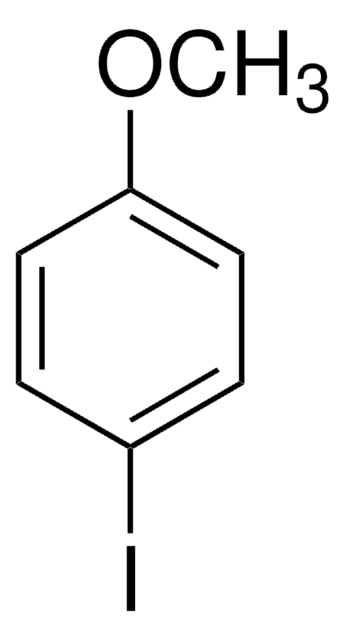
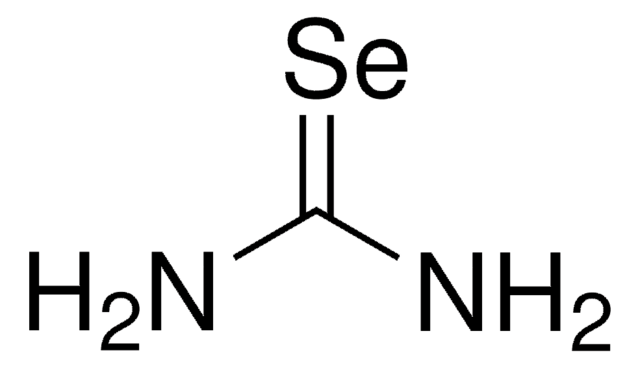
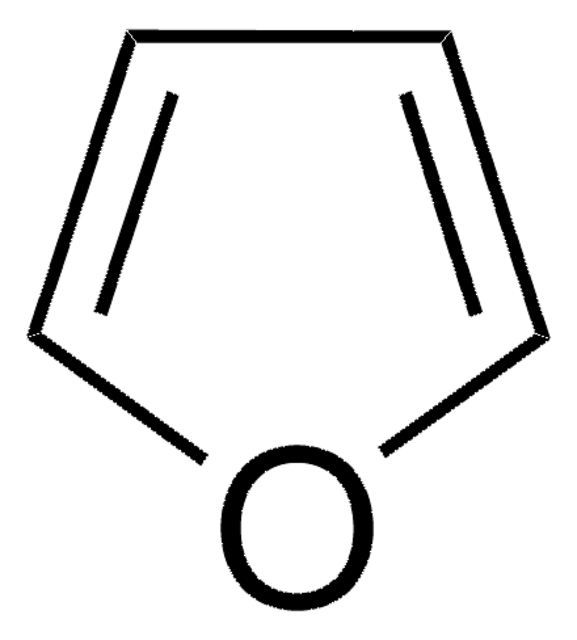
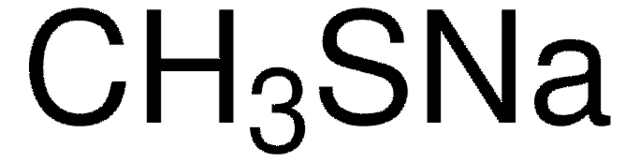추천 제품
Quality Level
분석
97%
양식
liquid
refractive index
n20/D 1.58 (lit.)
bp
110-111 °C (lit.)
mp
−30 °C (lit.)
density
1.423 g/mL at 25 °C (lit.)
SMILES string
c1cc[se]c1
InChI
1S/C4H4Se/c1-2-4-5-3-1/h1-4H
InChI key
MABNMNVCOAICNO-UHFFFAOYSA-N
일반 설명
Selenophene is a heterocyclic building block. Synthesis of selenophene-based heteroacenes has been reported. Synthesis of selenophene-thiophene block copolymers has been reported. The electron transmission spectra of selenophene has been recorded in the 0-6eV energy range.
애플리케이션
Selenophene may be used:
- as conjugated linker in the synthesis of organic dyes
- in the synthesis of selenophene-2-carbonitrile
- in the synthesis of selenophene diketopyrrolopyrrole polymers
- as building block for the electrically conducting polyalkyl selenophene
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2 - STOT RE 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
26.6 °F - closed cup
Flash Point (°C)
-3 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Journal of the Electrochemical Society, 137, 1827-1827 (1990)
Low band gap selenophene-diketopyrrolopyrrole polymers exhibiting high and balanced ambipolar performance in bottom-gate transistors.
Shahid M, et al.
Chemical Science, 3(1), 181-185 (2012)
Dye-sensitized solar cells based on organic sensitizers with different conjugated linkers: furan, bifuran, thiophene, bithiophene, selenophene, and biselenophene.
Li R, et al.
The Journal of Physical Chemistry C, 113(17), 7469-7479 (2009)
Electron transmission spectra of selenophene and tellurophene and Xa computations of electron affinities for chalcophenes.
Modelli A, et al.
Chemical Physics, 88(3), 181-185 (1984)
Toshihiro Okamoto et al.
Organic letters, 7(23), 5301-5304 (2005-11-05)
[reaction: see text] A new intramolecular triple cyclization of bis(o-haloaryl)diacetylenes, via dilithiation followed by reaction with chalcogen elements, produces pi-conjugated compounds containing heterole-1,2-dichalcogenin-heterole fused tricyclic skeletons. The subsequent dechalcogenation with copper metal affords a series of thiophene- and selenophene-based heteroacenes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.