추천 제품
Quality Level
분석
98%
형태
solid
광학 활성
[α]20/D +11°, c = 6 in chloroform
mp
126-128 °C (lit.)
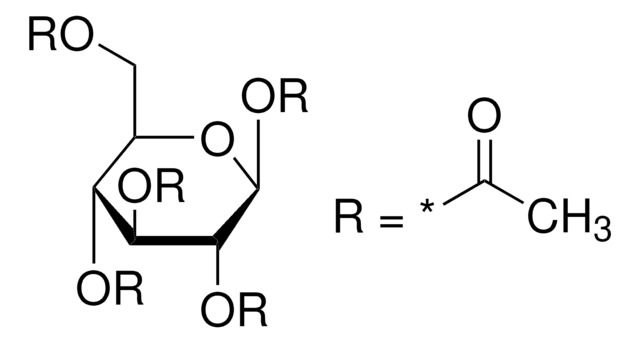
SMILES string
CC(=O)O[C@@H]1O[C@H](CO)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O
InChI
1S/C14H20O10/c1-6(16)20-11-10(5-15)24-14(23-9(4)19)13(22-8(3)18)12(11)21-7(2)17/h10-15H,5H2,1-4H3/t10-,11-,12+,13-,14-/m1/s1
InChI key
FEQXFAYSNRWXDW-RKQHYHRCSA-N
일반 설명
1,2,3,4-Tetra-O-acetyl-β-ᴅ-glucopyranoseis a carbohydrate that is used in the synthesis of disaccharides and D-glucose6-phosphate.
애플리케이션
Phosphorylated derivatives have proven valuable in the study of substrates for inositol synthase, and for the preparation of anionic surfactants.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Beata Liberek et al.
Carbohydrate research, 341(13), 2275-2285 (2006-07-15)
The single-crystal X-ray diffraction and high-resolution 1H and 13C NMR spectral data for methyl 2,5-di-O-acetyl-beta-D-glucofuranosidurono-6,3-lactone and 1,2,5-tri-O-acetyl-beta-D-glucofuranurono-6,3-lactone are reported. The lactones were synthesized as byproducts of reactions carried out to obtain methyl 1,2,3,4-tetra-O-acetyl-D-glucopyranuronate. The conformations of these lactones in the
Yuriko Y Root et al.
Carbohydrate research, 337(21-23), 2343-2346 (2002-11-16)
The identity of the crystalline product formed by the acetylation of a mixture of methyl alpha- and beta-D-glucopyranuronates has been confirmed as being methyl 1,2,3,4-tetra-O-acetyl-beta-D-glucopyranuronate (3), which agrees with the assignment from 1H NMR. The absolute configuration of compound 3
A Milius et al.
Carbohydrate research, 229(2), 323-336 (1992-05-22)
D-Glucose 3- and 6-[sodium 2-(perfluoro-hexyl or -octyl)ethyl phosphates) have been synthesized by condensation of 1,2,3,4,-tetra-O-acetyl-beta-D-glucopyranose and 1,2:5,6-di-O-isopropylidene-alpha-D-glucofuranose with 2-(perfluoroalkyl)ethylphosphoroditriazolides followed by O-deacetylation or deacetalation. The structures of the compounds were established on the basis of 1H-, 19F-, 31P-, and 13C-NMR
Tetrahedron, 47, 3895-3895 (1991)
Wei Wang et al.
Glycoconjugate journal, 31(2), 133-143 (2013-11-13)
A group of fluorescent statistical glycopolymers, prepared via reversible addition-fragmentation chain-transfer (RAFT)-based polymerizations, were successfully employed in lectin-mediated bacterial binding studies. The resultant glycopolymers contained three different monomers: N-(2-hydroxyethyl) acrylamide (HEAA), N-(2-aminoethyl) methacrylamide (AEMA) and N-(2-glyconamidoethyl)-methacrylamides possessing different pendant sugars.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.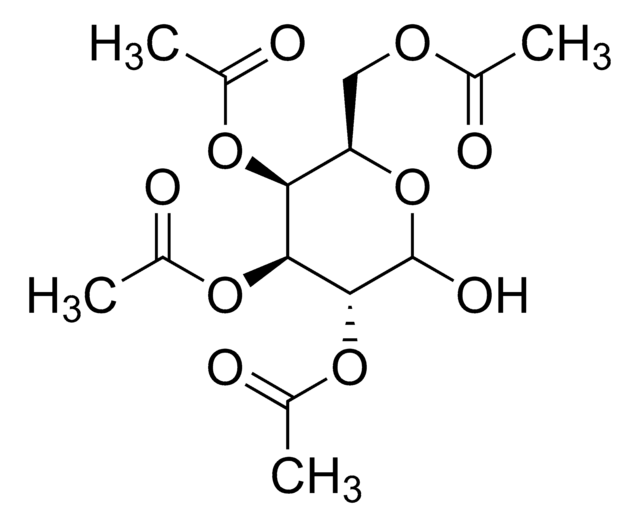



![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)