추천 제품
Quality Level
분석
97%
양식
powder
환경친화적 대안 제품 점수
old score: 18
new score: 7
Find out more about DOZN™ Scoring
환경친화적 대안 제품 특성
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
103-106 °C (lit.)
작용기
carboxylic acid
imide
maleimide
환경친화적 대안 카테고리
저장 온도
2-8°C
SMILES string
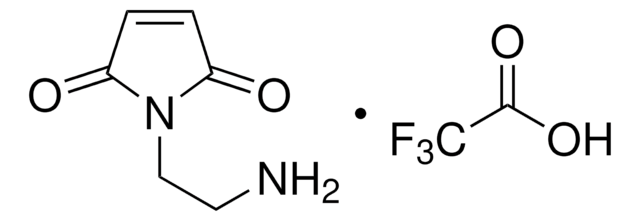
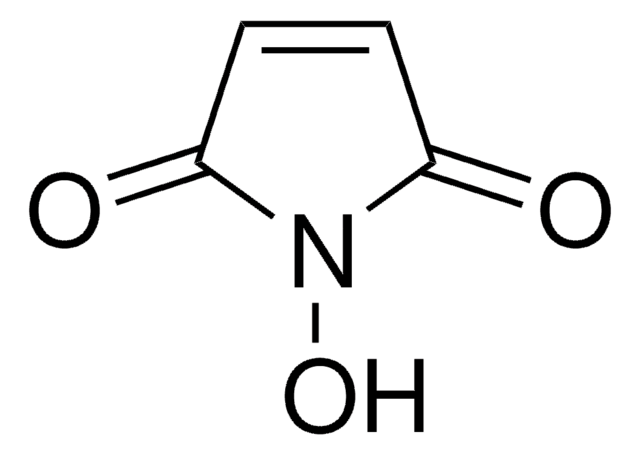
OC(=O)CCN1C(=O)C=CC1=O
InChI
1S/C7H7NO4/c9-5-1-2-6(10)8(5)4-3-7(11)12/h1-2H,3-4H2,(H,11,12)
InChI key
IUTPJBLLJJNPAJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
N-Maleoyl-β-alanine (3-maleimidopropanoic acid) is an aliphatic N-substituted maleimide. It is one of the component of the stabilizing solution for EC145 (a folate-targeted vinca alkaloid conjugate) used in rodent pharmacokinetic studies.
We are committed to bringing you Greener Alternative Products, which adhere to one of the four categories of Greener Alternatives . This product belongs to category of Re-engineered products, showing key improvements in Green Chemistry Principles “Less Hazardous Chemical Syntheses”, "Safer Solvents and Auxiliaries" and “Inherently Safer Chemistry for Accident Prevention”. Click here to view its DOZN scorecard.
애플리케이션
N-Maleoyl-β-alanine (3-maleimidopropanoic acid, N-(2-carboxyethyl)maleimide)) is the suitable reagent used in the following studies:
- To decrease the biotin binding affinity of Avd(S16C) (avidin with a single point mutation S16C).
- As a side chain reactive agent to modify tryptic peptides that result in mass shifts indicating the presence of cysteine residues.
- To preblock Xenopus laevis oocytes for exposed cysteines used as an expression system in the study of conformational changes in cASIC1a Receptors.
- As a protective agent for keratin fiber in high temperature process.
- As a non-cleavable maleimido moiety during the synthesis of tetrawalled molecular umbrella-octaarginine conjugates.
- Synthesis of organotin carboxylates of N-Maleoyl-β-alanine.
- To functionalize the gold surfaces to interact with cysteine-modified peptide.
- Preparation of cross-linked dextran–poly(ethylene glycol) hydrogel substrate.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Christopher P Leamon et al.
The Journal of pharmacology and experimental therapeutics, 336(2), 336-343 (2010-10-28)
During a phase I trial of EC145 (a folate-targeted vinca alkaloid conjugate), constipation was identified as the dose-limiting toxicity, probably from a nonfolate receptor-related liver clearance process capable of releasing unconjugated vinca alkaloid from EC145 and shuttling it to the
A new strategy for the preparation of maleimide-functionalised gold surfaces.
Electrochemistry Communications 12.10 (2010): 1403-1406.
Electrochemistry Communications 12.10 (2010): 1403-1406.
Zhang X, et al
Electrochemical Communications, 12(10), 1403-1406 (2010)
Organotin (IV) derivatives of N-maleoylamino acids: their synthesis and structural elucidation.
Bhatti MH, et al
Turkish Journal of Chemistry, 29(5), 463-476 (2005)
Protecting keratin fiber with water soluble N-substituted maleimides in high temperature processes.
Cai JY, et al.
Fibers and Polymers, 15(11), 2247-2252 (2014)
Swarna S Ramaswamy et al.
The Journal of biological chemistry, 288(50), 35896-35903 (2013-11-08)
Acid-sensing ion channels are cation channels activated by external protons and play roles in nociception, synaptic transmission, and the physiopathology of ischemic stroke. Using luminescence resonance energy transfer (LRET), we show that upon proton binding, there is a conformational change
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.