407275
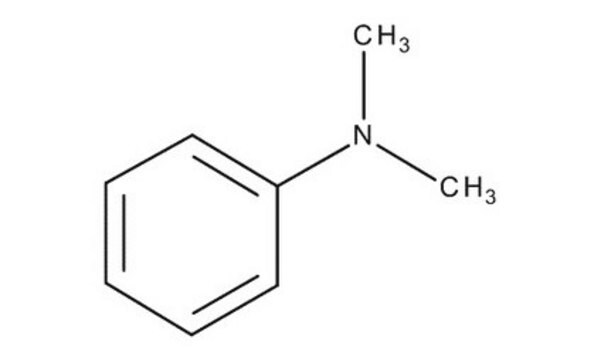
N,N-Dimethylaniline
purified by redistillation, ≥99.5%
동의어(들):
N,N-Dimethylaniline, N,N-Dimethylphenylamine, DMA, Dimethylaniline, Dimethylphenylamine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5N(CH3)2
CAS Number:
Molecular Weight:
121.18
Beilstein:
507140
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥99.5%
형태
liquid
정제법
glass distillation
redistillation
refractive index
n20/D 1.557 (lit.)
pH
7.4 (20 °C, 1.2 g/L)
bp
193-194 °C (lit.)
mp
1.5-2.5 °C (lit.)
density
0.956 g/mL at 25 °C (lit.)
작용기
amine
SMILES string
CN(C)c1ccccc1
InChI
1S/C8H11N/c1-9(2)8-6-4-3-5-7-8/h3-7H,1-2H3
InChI key
JLTDJTHDQAWBAV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Anodic oxidation of N,N-dimethylaniline at platinum electrode in acidic buffers has been studied by conventional and cyclic voltammetry. Photoreduction of excited benzophenone (BP) by DMA in acetonitrile solution has been investigated by picosecond-femtosecond laser photolysis and time resolved transient absorption spectroscopy.
애플리케이션
N,N-Dimethylaniline (DMA) may be used for the preparation of poly(N,N-dimethylaniline) film by anodic oxidation of DMA.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Carc. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
167.0 °F - closed cup
Flash Point (°C)
75 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Femtosecond-picosecond laser photolysis studies on photoreduction process of excited benzophenone with N,N-dimethylaniline in acetonitrile solution.
Miyasaka H, et al.
Bulletin of the Chemical Society of Japan, 63(12), 3385-3397 (1990)
Electrochemically polymerized N, N-dimethylaniline film with ion-exchange properties as an electrode modifier.
Oyama N, et al.
Analytical Chemistry, 57(8), 1526-1532 (1985)
Anodic oxidation studies of N,N-dimethylaniline. I. Voltammetric and spectroscopic investigations at platinum electrodes.
Mizoguchi T and Adams RN.
Journal of the American Chemical Society, 84(11), 2058-2061 (1962)
Kenji Hirai et al.
Chemical communications (Cambridge, England), 48(52), 6472-6474 (2012-04-21)
Spatiospecific functionalisation of a shell crystal was performed in a core-shell crystal of a porous coordination polymer (PCP) via post-synthetic modification (PSM). The shell crystal allowed the core crystal to selectively accumulate N,N-dimethylaniline (DMA) and afford the intense exciplex fluorescence.
Molecular fibers and wires in solid-state and solution self-assemblies of cyclodextrin [2]rotaxanes.
Subashani Maniam et al.
Organic letters, 10(10), 1885-1888 (2008-04-16)
Cyclodextrin [2]rotaxanes have been prepared by coupling dimethylanilines with dicarboxylic acids using DMT-MM, in aqueous solutions of alpha-cyclodextrin, and the example illustrated shows unusual fluorescence emission and other spectroscopic behavior characteristic of the formation of molecular wires in solution, similar
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.