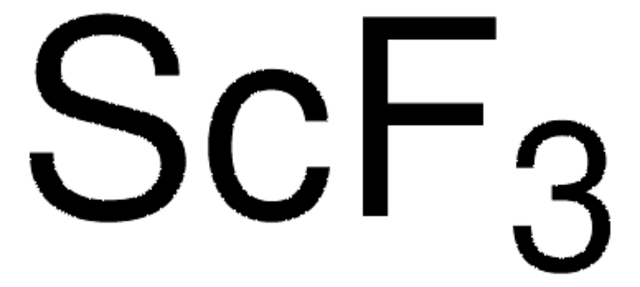추천 제품
Grade
anhydrous
Quality Level
분석
99.9% trace metals basis
형태
powder
반응 적합성
reagent type: catalyst
core: scandium
불순물
≤1500.0 ppm Trace Rare Earth Analysis
mp
960 °C (lit.)
density
2.39 g/mL at 25 °C (lit.)
SMILES string
Cl[Sc](Cl)Cl
InChI
1S/3ClH.Sc/h3*1H;/q;;;+3/p-3
InChI key
DVMZCYSFPFUKKE-UHFFFAOYSA-K
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Scandium(III) chloride (ScCl3) is a scandium halide that can be formed by dissolving scandium oxide in an acid solution and evaporating the resultant solution to dryness.
애플리케이션
ScCl3 can be used in the preparation of lithium salt of anionic scandium tetraborohydride complex (LiSc(BH4)4) by forming a ball-milled mixture. LiSc(BH4)4 can be used as a potential hydrogen storage material.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
LiSc (BH4) 4 as a hydrogen storage material: multinuclear high-Resolution solid-state NMR and first-Principles Density functional Theory Studies
Kim C, et al.
The Journal of Physical Chemistry C, 113(22), 9956-9968 (2009)
Preparation and mechanism of formation of anhydrous scandium (III) chloride and bromide
Stotz RW and Melson GA
Inorganic Chemistry, 11(7), 1720-1721 (1972)
문서
Rare earth elements are vital in everyday life worldwide: catalysts in cars, colors in screens, magnets in electronics. Essential for modern living.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.