418218
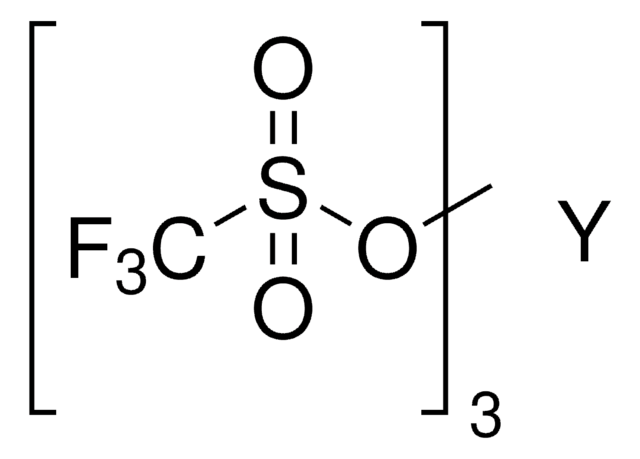
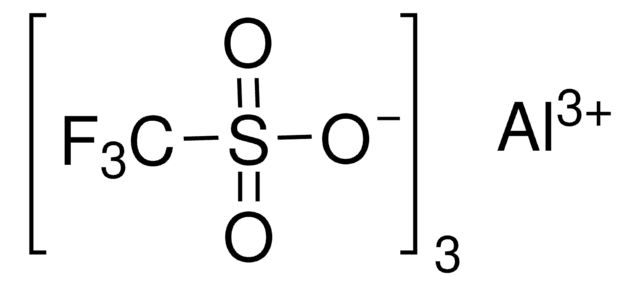
Scandium(III) triflate
99%
동의어(들):
Sc(OTf)3, Scandium(III) trifluoromethanesulfonate, Trifluoromethanesulfonic acid scandium(III) salt
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
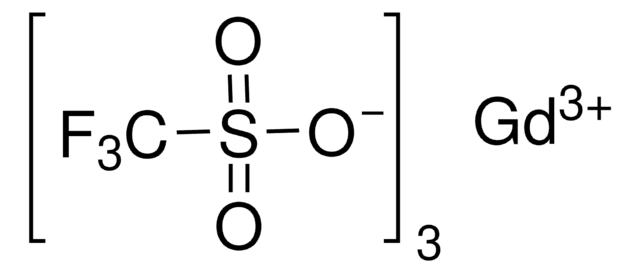
Sc(SO3CF3)3
CAS Number:
Molecular Weight:
492.16
Beilstein:
8510151
MDL number:
UNSPSC 코드:
12161600
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
양식
powder
반응 적합성
core: scandium
reagent type: catalyst
SMILES string
[Sc+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
HZXJVDYQRYYYOR-UHFFFAOYSA-K
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates.
애플리케이션
Scandium(III) triflate was used as a catalyst in:
- Hydrothiolation reaction of aromatic and aliphatic thiols.
- Selective two-electron reduction of O2 by ferrocene derivatives.
- Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
- Synthesis of β-cyanoketones.
- Combination with triethylsilane to reductively open functionalized pyranoside rings.
- The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Alex R Lippert et al.
Journal of the American Chemical Society, 128(46), 14738-14739 (2006-11-16)
Oligosubstituted bullvalones were synthesized in eight steps from 2,6-cycloheptadienone via a unique Lewis acid catalyzed intramolecular cyclopropanation of a stabilized sulfur ylide, leading directly to the tetracyclic cage structure. Upon exposure to base, the substituted bullvalones tautomerized to a hydroxybullvalene
Saya Kakuda et al.
Journal of the American Chemical Society, 137(9), 3330-3337 (2015-02-11)
Mononuclear copper complexes, [(tmpa)Cu(II)(CH3CN)](ClO4)2 (1, tmpa = tris(2-pyridylmethyl)amine) and [(BzQ)Cu(II)(H2O)2](ClO4)2 (2, BzQ = bis(2-quinolinylmethyl)benzylamine)], act as efficient catalysts for the selective two-electron reduction of O2 by ferrocene derivatives in the presence of scandium triflate (Sc(OTf)3) in acetone, whereas 1 catalyzes
Okamoto, Y., et al.
Macromolecular Symposia, 183, 83-83 (2002)
Giovanni Desimoni et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(12), 3630-3636 (2008-02-29)
The asymmetric Friedel-Crafts reaction between a series of substituted indoles 2 a-l and methyl (E)-2-oxo-4-aryl-3-butenoates 3 a-c has been efficiently catalyzed by the scandium(III) triflate complex of (4'S,5'S)-2,6-bis[4'-(triisopropylsilyl)oxymethyl-5'-phenyl-1',3'-oxazolin-2'-yl]pyridine (pybox; 1). Substituted 4-(indol-3-yl)-2-oxo-4-arylbutyric acid methyl esters 4 a-n were usually formed
Mild reductive opening of aryl pyranosides promoted by scandium(III) triflate.
Hua-Li Qin et al.
Journal of the American Chemical Society, 129(1), 38-39 (2007-01-04)
문서
Friedel-Crafts acylation with Lewis acid catalysts forms monoacylated products via electrophilic aromatic substitution of arenes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.