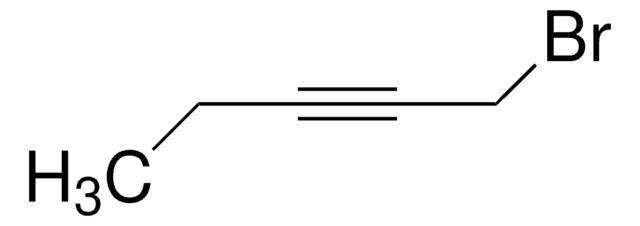427292
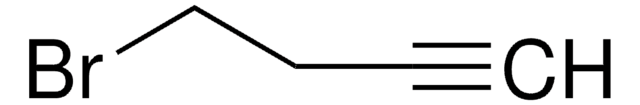
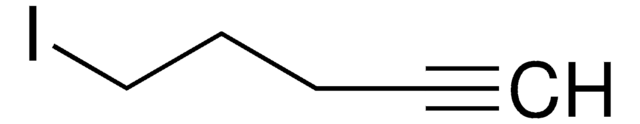
1-Bromo-2-butyne
≥98%
동의어(들):
1-(Bromomethyl)-2-methylacetylene, 2-Butyn-1-yl bromide, 2-Butynyl bromide, 4-Bromobut-2-yne
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
CH3C≡CCH2Br
CAS Number:
Molecular Weight:
132.99
Beilstein:
605306
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
1-Bromo-2-butyne is a propargyl bromide derivative. It is one of the constitutional isomer of bromo butyne. Its Br-loss threshold photoionization breakdown diagram has been analyzed to derive dissociative photoionization thresholds to C4H5+ production. It participates in the preparation of linagliptin.
애플리케이션
1-Bromo-2-butyne was used in the alkylation of L-tryptophan methyl ester. It was used as a source to generate CH3CCCH2 radicals to investigate the reaction kinetics of these radicals with NO and NO2.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 4-butynyloxybenzene sulfonyl chloride
- mono-propargylated diene derivative
- isopropylbut-2-ynylamine
- allenylcyclobutanol derivatives
- allyl-[4-(but-2-ynyloxy)phenyl]sulfane
- allenylindium
- alkynyl alcohols
- axially chiral teranyl compounds
Exploited in the synthesis of axially chiral teranyl compounds.
이미 열람한 고객
Exploring the functional space of thiiranes as gelatinase inhibitors using click chemistry.
Testero SA, et al.
ARKIVOC (Gainesville, FL, United States), 7, 221-236 (2011)
Facile Entry to 4, 5, 6, 7-Tetrahydro [1, 2, 3] triazolo [1, 5-a] pyrazin-6-ones from Amines and Amino Acids.
Sai SV, et al.
European Journal of Organic Chemistry, 2008(14), 2423-2429 (2008)
Imaging breakdown diagrams for bromobutyne isomers with photoelectron-photoion coincidence.
Bodi A and Hemberger P.
Physical Chemistry Chemical Physics, 16(2), 505-515 (2014)
Takanori Shibata et al.
Journal of the American Chemical Society, 126(27), 8382-8383 (2004-07-09)
An asymmetric [2+2+2] cycloaddition of an alpha,omega-diyne, possessing ortho-substituted aryl groups on its terminus, and a monoalkyne with oxygen functionalities gave various axially chiral teraryl compounds. The coupling proceeded with extremely high enantio- (>99.5% ee) and diastereoselectivities (dl/meso = >95/5)
Acetylenic TACE inhibitors. Part 1. SAR of the acyclic sulfonamide hydroxamates.
Levin JI, et al.
Bioorganic & Medicinal Chemistry Letters, 13(16), 2799-2803 (2003)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.