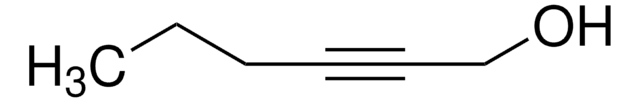추천 제품
Quality Level
분석
97%
형태
liquid
refractive index
n20/D 1.441 (lit.)
bp
128.9 °C (lit.)
mp
−63.6 °C (lit.)
density
0.926 g/mL at 25 °C (lit.)
작용기
hydroxyl
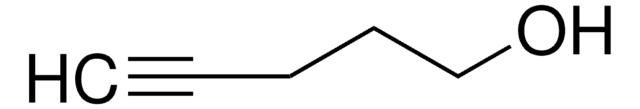
SMILES string
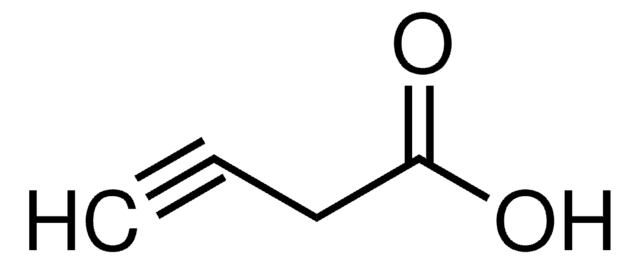
OCCC#C
InChI
1S/C4H6O/c1-2-3-4-5/h1,5H,3-4H2
InChI key
OTJZCIYGRUNXTP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The enthalpies of combustion and vaporization of 3-butyn-1-ol is measured by static bomb combustion calorimetry and correlation gas chromatography techniques.
애플리케이션
3-Butyn-1-ol was used to prepare Markovnikov addition product.
Alkynyl substrate used in a study of a palladium-catalyzed coupling with β-tetrionic acid bromide leading to alkynyl substituted furanones in good yield.
Synthon for preparation of oxygen-containing heterocycles and protected esters of s-Hydroxy-L-isoleucine
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
96.8 °F - closed cup
Flash Point (°C)
36 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Kopf, Henning; Pietraszuk. et al.
Organometallics, 25(10), 2533-2546 (2006)
Tetrahedron Letters, 48, 105-105 (2007)
Ederley Vélez et al.
The journal of physical chemistry. A, 109(34), 7832-7838 (2006-07-13)
The enthalpies of combustion and vaporization of 3-buten-1-ol and 3-butyn-1-ol have been measured by static bomb combustion calorimetry and correlation gas chromatography techniques, respectively, and the gas-phase enthalpies of formation, Delta(f)H degrees (m)(g), have been determined, the values being -147.3
Deniz Dogruer et al.
Bioorganic & medicinal chemistry letters, 14(2), 523-526 (2003-12-31)
The binding of a series pyridylbutynylamines 6 was examined at alpha4beta2 nACh receptors. Structural modifications, comparing 6 with pyridyl ethers 2, did not consistently result in parallel effects on receptor affinity, suggesting possible differences in their modes of binding. Furthermore
Kristen Bennett et al.
Applied and environmental microbiology, 82(8), 2270-2279 (2016-01-31)
Nitrosomonas europaea is an aerobic nitrifying bacterium that oxidizes ammonia (NH3) to nitrite (NO2 (-)) through the sequential activities of ammonia monooxygenase (AMO) and hydroxylamine dehydrogenase (HAO). Many alkynes are mechanism-based inactivators of AMO, and here we describe an activity-based
문서
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.