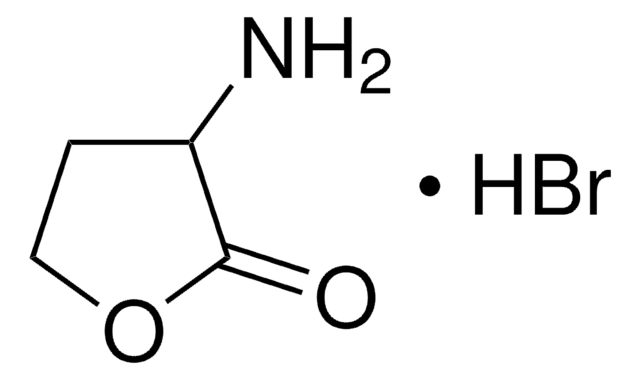
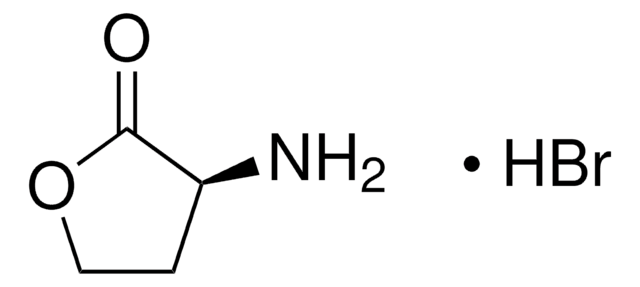
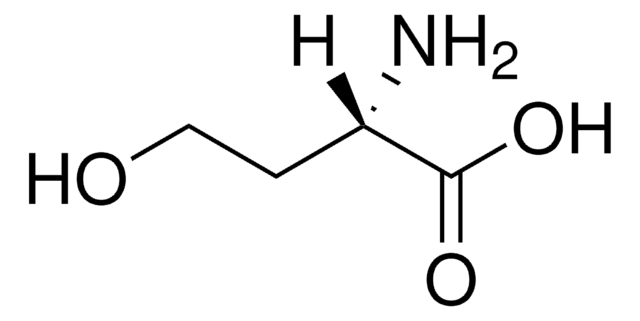
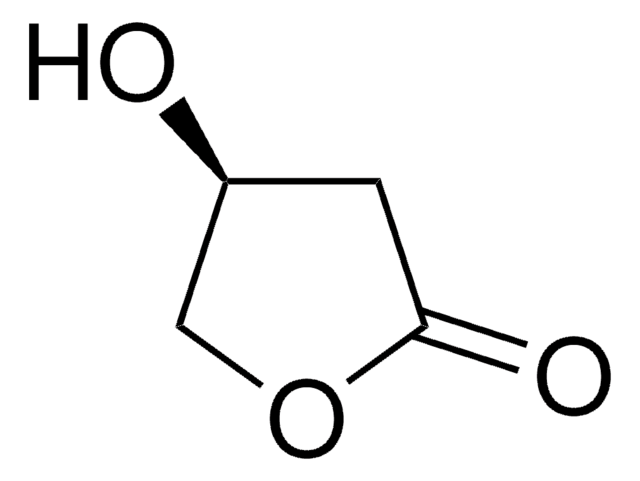
추천 제품
애플리케이션
(S)-α-Amino-γ-butyrolactone hydrochloride can be used:
- As a precursor for the preparation of amino-keto-alcohols and β-amino acids.
- To prepare N-acylhomoserine lactone (AHL) analogs by reacting with substituted 2-chloro-N-phenylacetamide and different halides.
- As a starting material for the synthesis of L-discadenine.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Natural products from social amoebae
Barnett R and Stallforth P
Chemistry?A European Journal , 24(17), 4202-4214 (2018)
Design, synthesis and antibacterial evaluation of novel AHL analogues
Ren J, et al.
Bioorganic & Medicinal Chemistry Letters, 23(14), 4154-4156 (2013)
Reaction of (S)-homoserine lactone with Grignard reagents: synthesis of amino-keto-alcohols and β-amino acid derivatives
Gundogdu O, et al.
Tetrahedron Asymmetry, 28(9), 1163-1168 (2017)
L R Brunham et al.
The pharmacogenomics journal, 14(6), 555-563 (2014-05-28)
Differences in the frequency of pharmacogenomic variants may influence inter-population variability in drug efficacy and risk of adverse drug reactions (ADRs). We investigated the diversity of ∼ 4500 genetic variants in key drug-biotransformation and -response genes among three South East
Cheng-Siang Wong et al.
World journal of microbiology & biotechnology, 28(2), 453-461 (2012-07-19)
A chemically defined medium called KGm medium was used to isolate from a sample of sea water a bacterial strain, MW3A, capable of using N-3-oxohexanoyl-L: -homoserine lactone as the sole carbon source. MW3A was clustered closely to Pseudomonas aeruginosa by
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.