모든 사진(2)
About This Item
실험식(Hill 표기법):
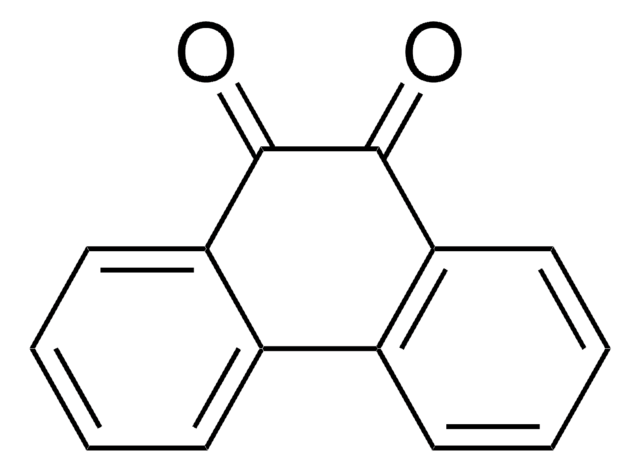
C12H6N2O2
CAS Number:
Molecular Weight:
210.19
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
260 °C (dec.) (lit.)
작용기
ketone
SMILES string
O=C1C(=O)c2cccnc2-c3ncccc13
InChI
1S/C12H6N2O2/c15-11-7-3-1-5-13-9(7)10-8(12(11)16)4-2-6-14-10/h1-6H
InChI key
KCALAFIVPCAXJI-UHFFFAOYSA-N
관련 카테고리
일반 설명
1,10-Phenanthroline-5,6-dione (phendio) forms Cu(II) and Ag(I) phendio complexes, which show potent anti-fungal and anti-cancer activity. The modification of glassy carbon (GC) electrodes with phendio complexes of transition metals leads to the catalytic oxidation of NADH at low overpotential.
애플리케이션
1,10-Phenanthroline-5,6-dione may be used in the preparation of homo- and heterometallic complexes with early transition metal ions.
A Bifunctional quinone oxidant which, when used in conjunction with Zn2+ catalysts, is used to affect the aerobic oxidation of secondary amines to a variety of value added motifs, including indoles.
Bioinspired Aerobic Oxidation of Secondary Amines and Nitrogen Heterocycles with a Bifunctional Quinone Catalyst
Bioinspired Aerobic Oxidation of Secondary Amines and Nitrogen Heterocycles with a Bifunctional Quinone Catalyst
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Yu-Min Song et al.
Journal of inorganic biochemistry, 103(3), 396-400 (2009-01-13)
Three new solid complexes have been synthesized by the reaction of rare earth(III) nitrate with the first ligand curcumin (HL) and the second ligand 1,10-phenanthroline-5,6-dione (L') in alcohol solution (pH=6.5-7.0). The composition of the complexes has been characterized by elemental
Electrocatalytic oxidation of NADH at glassy carbon electrodes modified with transition metal complexes containing 1, 10-phenanthroline-5, 6-dione ligands.
Wu Q, et al.
Analytical Chemistry, 68(20), 3688-3696 (1996)
1, 10-Phenanthroline-5, 6-dione as a building block for the synthesis of homo-and heterometallic complexes.
Calderazzo F, et al.
Inorgorganica Chimica Acta, 330(1), 136-142 (2002)
E Lorenzo et al.
Biosensors & bioelectronics, 13(3-4), 319-332 (1998-06-27)
Various strategies based on the use of chemically modified electrodes for the development of amperometric biosensors are described. Particular emphasis is placed on materials capable of catalyzing the oxidation of NADH and coupling these with enzymatic activities for biosensor construction.
Malachy McCann et al.
Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine, 17(6), 635-645 (2005-02-04)
The Cu(II) and Ag(I) complexes, [Cu(phendio)3](ClO4)2 x 4H2O and [Ag(phendio)2]ClO4 (phendio = 1,10-phenanthroline-5,6-dione), are prepared in good yield by reacting phendio with the appropriate metal perchlorate salt. The X-ray crystal structure of the Ag(I) complex shows it to have a
관련 콘텐츠
he Stahl Lab focuses on the development of catalysts and catalytic reactions for selective oxidation of organic molecules, with particular emphasis on aerobic oxidation reactions.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.