633348
Vinylboronic acid pinacol ester
contains phenothiazine as stabilizer, 95%
동의어(들):
2-Ethenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 2-Vinyl-4,4,5,5-tetramethyl-1,3,2-dioxaoborolane, 4,4,5,5-Tetramethyl-2-vinyl-1,3,2-dioxaborolane
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
실험식(Hill 표기법):
C8H15BO2
CAS Number:
Molecular Weight:
154.01
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
포함
phenothiazine as stabilizer
refractive index
n20/D 1.4300 (lit.)
density
0.908 g/mL at 25 °C (lit.)
저장 온도
−20°C
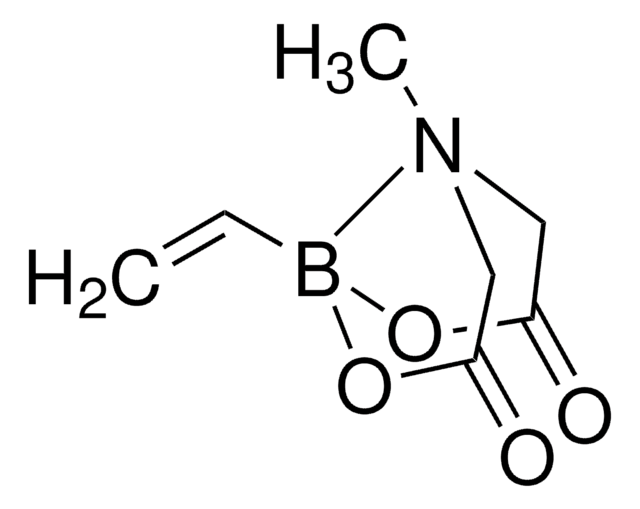
SMILES string
CC1(C)OB(OC1(C)C)C=C
InChI
1S/C8H15BO2/c1-6-9-10-7(2,3)8(4,5)11-9/h6H,1H2,2-5H3
InChI key
DPGSPRJLAZGUBQ-UHFFFAOYSA-N
애플리케이션
Employed in a "double" Heck-Mizoroki arylation leading to β,β-diarylated vinyl boronates which react with an additional aryl halide to form Π-extended systems. This approach was used to prepare conjugated dendrimers. Also used to prepare γ-carbonyl vinyl boronates via a light-induced radical addition of xanthates.
Reagent used for
Reagent used in Preparation of
- Suzuki-Miyaura coupling reactions
- Mizoroki-Heck reactions (cascade reaction)
- Intramolecular Nozaki-Hiyama-Kishi reactions
- Stereoselective Cu-catalyzed γ-selective and stereospecific coupling
- Control of stereoselectivity and mechanistic portrait on intramolecular (4+1)-cycloaddition of dialkoxycarbenes
- Regio- and stereoselective synthesis of trisubstituted alkenes via gold(I)-catalyzed hydrophosphoryloxylation of haloalkynes followed by Pd-catalyzed consecutive cross-coupling reactions
- Asymmetric Birch reductive alkylation
Reagent used in Preparation of
- Molecular tubes for lipid sensing
- Enzymatic inhibitors, antibiotics, receptor analogs, and other biologically significant compounds (including total syntheses)
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 2 - Flam. Liq. 3 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
93.2 °F
Flash Point (°C)
34 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Bathoju Chandra Chary et al.
Chemical communications (Cambridge, England), 47(27), 7851-7853 (2011-06-07)
A new stereoselective synthesis of trisubstituted alkenes is developed. Hydrophosphoryloxylation of haloalkynes provides Z-alkenyl halophosphates, which undergo Pd-catalyzed consecutive cross-coupling reactions to afford regio- and stereodefined trisubstituted alkenes.
Christopher A Leclair et al.
Tetrahedron letters, 51(52), 6852-6855 (2011-04-26)
A total synthesis of LL-Z1640-2 (2), a potent and selective kinase inhibitor, has been completed. The key step of the convergent synthesis utilized a late-stage intramolecular Nozaki-Hiyama-Kishi (NHK) reaction to close the macrocycle at the C6'-C7' bond.
Francis Beaumier et al.
Journal of the American Chemical Society, 134(13), 5938-5953 (2012-03-13)
The stereoselective synthesis of 5-5, 6-5, and 7-5 fused O-heterocyclic compounds is reported. The key reaction is a formal intramolecular (4 + 1)-cycloaddition involving a dialkoxycarbene and an electron-deficient diene where the stereoselectivity is dependent on the length of the
Markus R Heinrich et al.
Chemical communications (Cambridge, England), (24), 3077-3079 (2005-06-17)
Gamma-carbonyl vinyl boronates can be prepared by a visible light induced radical chain addition of an S-acyl dithiocarbonate (xanthate) to the pinacol ester of vinyl boronic acid, followed by treatment with base.
Mingyu Yang et al.
Organic letters, 14(3), 816-819 (2012-01-20)
A Cu-catalyzed γ-selective coupling reaction between propargylic phosphates and aryl- or alkenylboronates afforded aryl- or alkenyl-conjugated allenes. The reaction showed excellent functional group compatibility in both the propargylic substrates and the boronates. The reaction of an enantioenriched propargylic phosphate proceeded
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)