271438
Tributyl(vinyl)tin
97%
동의어(들):
Tributyl(vinyl)stannane, Tributylstannylethylene, Vinyltributylstannane
로그인조직 및 계약 가격 보기
모든 사진(6)
About This Item
Linear Formula:
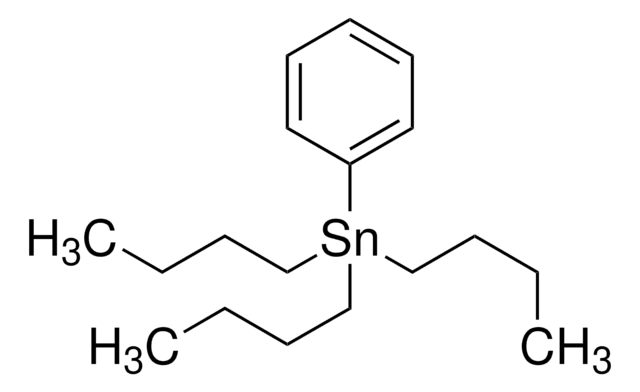
CH2=CHSn[CH3(CH2)3]3
CAS Number:
Molecular Weight:
317.10
Beilstein:
3537662
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
refractive index
n20/D 1.478 (lit.)
bp
104-106 °C/3.5 mmHg (lit.)
density
1.085 g/mL at 25 °C (lit.)
SMILES string
CCCC[Sn](CCCC)(CCCC)C=C
InChI
1S/3C4H9.C2H3.Sn/c3*1-3-4-2;1-2;/h3*1,3-4H2,2H3;1H,2H2;
InChI key
QIWRFOJWQSSRJZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Tributyl(vinyl)tin is an organostannane, commonly used in the palladium-catalyzed cross coupling reactions.
애플리케이션
Vinyl nucleophile for bromoacetylenes and bromoaromatics.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
109.4 °F - closed cup
Flash Point (°C)
43 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
The Journal of Organic Chemistry, 59, 7164-7164 (1994)
Ernesto G Occhiato et al.
Journal of medicinal chemistry, 47(14), 3546-3560 (2004-06-25)
New 5alpha-reductase 1 (5alphaR-1) inhibitors were designed to complete a consistent set of analogues suitable for a 3D QSAR study. These compounds were synthesized by a modification of the aza-Robinson annulation, further functionalized by Pd-catalyzed cross-coupling processes, and were tested
Masahiro Yoshida et al.
Organic letters, 6(12), 1979-1982 (2004-06-05)
[reaction: see text] A novel type of cascade ring expansion process has been developed by the palladium-catalyzed reaction of (Z)-1-(1,3-butadienyl)cyclobutanols with aryl iodides. The reaction proceeds in a stereospecific manner to produce (Z)-2-(3-aryl-1-propenyl)cyclopentanones. It has also been found that regioselective
New synthetic applications of organotin compounds: synthesis of stereodefined 2-iodo-2-alkenones, 2-substituted (E)-2-alkenones and 2-methyl-2-cycloalkenones
Fabio B et al.
Tetrahedron, 49, 4677-4698 (1993)
R Skoda-Földes et al.
Steroids, 60(12), 812-816 (1995-12-01)
Direct and carbonylative coupling reactions of various steroid derivatives possessing iodo- and bromo-alkenyl moiety (17-iodo-androst-16-ene, 1, 17-bromoandrost-2,16-diene, 2, 17-iodo-4-aza-4-methylandrost-16-en-3-one, 3, 17-iodo-4-azaandrost-16-en-3-one, 4) with vinyltributylstannane and ethynyltributylstannane were carried out in the presence of various palladium catalysts. While carbonylation took place
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)