추천 제품
Quality Level
분석
97%
반응 적합성
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings
mp
256-262 °C
작용기
phosphine
저장 온도
2-8°C
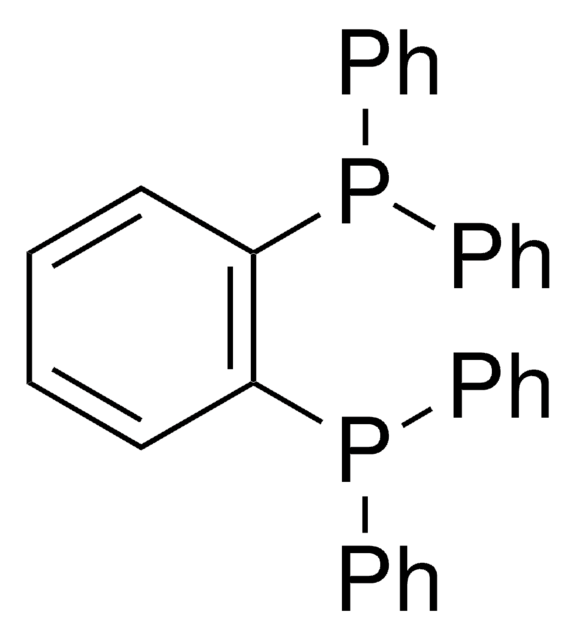
SMILES string
N1c2cccc(P(c3ccccc3)c4ccccc4)c2Oc5c1cccc5P(c6ccccc6)c7ccccc7
InChI
1S/C36H27NOP2/c1-5-15-27(16-6-1)39(28-17-7-2-8-18-28)33-25-13-23-31-35(33)38-36-32(37-31)24-14-26-34(36)40(29-19-9-3-10-20-29)30-21-11-4-12-22-30/h1-26,37H
InChI key
HSWZLYXRAOXOLL-UHFFFAOYSA-N
애플리케이션
N-XantPhos is a deprotonatable chelating aryldiphosphine ligand that can be used in:
- The preparation of Pd-NiXantphos catalyst system for the room temperature cross-coupling reactions of unactivated aryl chlorides.
- The synthesis of cinchonine iridium(III) cyclometalated complex that exhibits luminescence and good quantum efficiency.
- N-XantPhos and N-modified counterparts are also used in the preparation of rhodium based catalysts for hydroformylation reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
A new approach to dendritic supported NIXANTPHOS-based hydroformylation catalysts.
Ricken S, et al.
J. Mol. Catal. A: Chem., 257(1-2), 78-88 (2006)
NiXantphos: A Deprotonatable Ligand for Room-Temperature Palladium-Catalyzed Cross-Couplings of Aryl Chlorides.
Zhang J, et al.
Journal of the American Chemical Society, 136(17), 6276-6287 (2014)
Enhanced emission and analyte sensing by cinchonine iridium (III) cyclometalated complexes bearing bent diphosphine chelators.
Luo S X, et al.
Organometallics, 32(10), 2908-2917 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.








![Dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/374/597/f7932c5b-0448-498b-8254-f8ce1b9a4612/640/f7932c5b-0448-498b-8254-f8ce1b9a4612.png)