About This Item
추천 제품
Quality Level
분석
97%
양식
powder
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
152-155 °C (lit.)
SMILES string
[Pd].[Pd].O=C(\C=C\c1ccccc1)/C=C/c2ccccc2.O=C(\C=C\c3ccccc3)/C=C/c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;;/h3*1-14H;;/b3*13-11+,14-12+;;
InChI key
CYPYTURSJDMMMP-WVCUSYJESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
For small scale and high throughput uses, product is also available as ChemBeads (919772)
애플리케이션
- Application Guide for Palladium Catalyzed Cross-Coupling Reactions
- Synthesis of azepanes
- Synthesis of nanosized palladium phosphides upon interaction with white phosphorous
- Preparation of palladium triphenylphosphine carbonyl cluster complexes
- Precursor for synthesis of functionalized multiwalled carbon nanotube-palladium complexes used as catalysts for Heck coupling reactions
- Selective carbon-sulfur bond formation via addition of S-S and S-H bonds to alkynes
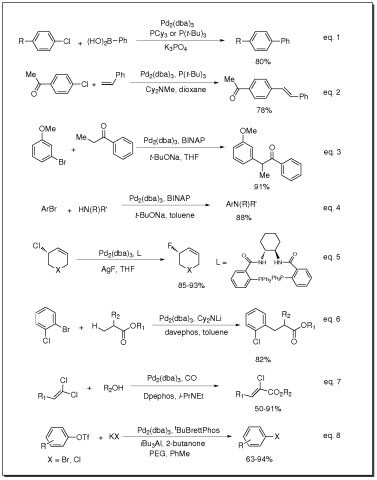
Reactant involved in:
- Catalyst for:
- Suzuki cross-coupling reactions
- PCN- and PCS-pincer palladium complex catalyzed tandem allylation
- Catalyst for Suzuki coupling of aryl chlorides (eq. 1)
- Catalyst for Heck coupling of aryl chlorides (eq. 2)
- Catalyst for arylation of ketones (eq. 3)
- Catalyst for Buchwald-Hartwig amination of aryl halides (eq. 4)
- Catalyst for fluorination of allylic chlorides (eq. 5)
- Catalyst for β-arylation of carboxylic esters (eq. 6)
- Catalyst for carbonylation of 1,1-dichloro-1-alkenes (eq. 7)
- Catalyst for conversion of aryl and vinyl triflates to aryl and vinyl halides (eq. 8)
- Pd source for enantioselective Tsuji Allylations
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
JosiPhos CyPF-tBu and palladium give catalyst for alkoxylation of activated heteroaryl halides with primary, secondary, and tertiary alcohols
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.







![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



