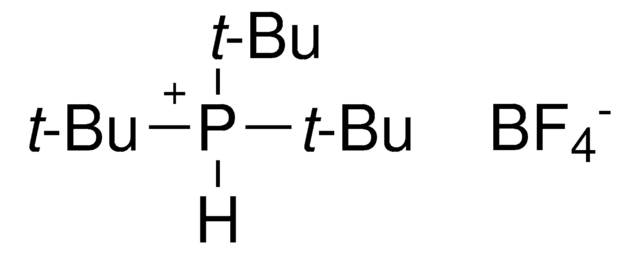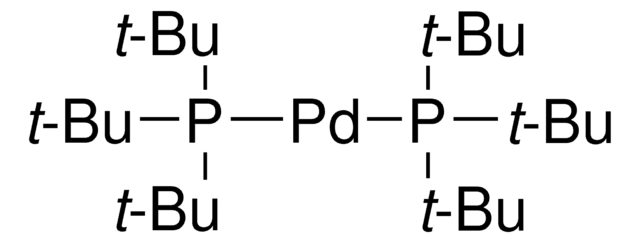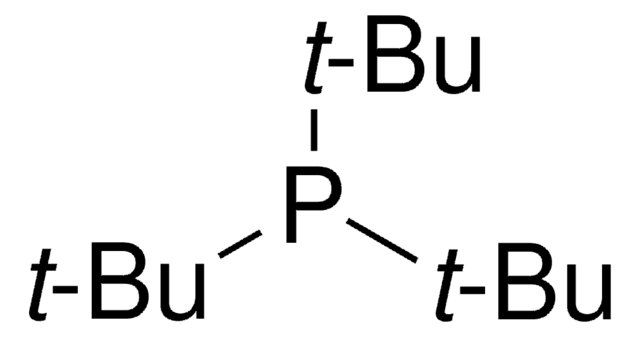추천 제품
형태
powder
Quality Level
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
SMILES string
[Pd].O=C(/C=C/c1ccccc1)\C=C\c2ccccc2.O=C(/C=C/c3ccccc3)\C=C\c4ccccc4
InChI
1S/2C17H14O.Pd/c2*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;/h2*1-14H;/b2*13-11+,14-12+;
InChI key
UKSZBOKPHAQOMP-SVLSSHOZSA-N
일반 설명
Bis(dibenzylideneacetone)palladium(0) (Pd(dba)2) is an air stable Pd0 complex. It is a reagent for the synthesis of allylic substituted cyclopentadienes. It is a homogeneous catalyst. It catalyzes the alkylation of allyl acetates by various nucleophiles under mild conditions.
애플리케이션
Bis(dibenzylideneacetone)palladium(0) (Pd(dba)2) was employed as catalyst in the following studies:
- Synthesis of isomeric 2-aryl-2,5-dihydrofurans, via Heck coupling of aryl bromides with alkenes using neopentyl phosphine ligands.
- Heck reaction of benzyl trifluoroacetate and 2,3-dihydrofuran phosphoramidite ligand.
- Allylation of stabilized anions.
- Cross coupling of allyl, alkenyl and aryl halides with organostannanes.
- Cross coupling of vinyl halides with alkenyl zinc species.
- Carbonylation of alkenyl and aryl halides.
- Employed with cyclic thiourea ligands in an efficient aerobic oxidation of alcohols to aldehydes and ketones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Cuerva, J. M.; Gomez-Bengoa, E.; et al
The Journal of Organic Chemistry, 62, 7540-7540 (1997)
Tetrahedron Letters, 22, 1399-1399 (1981)
Zhigang Yang et al.
Journal of the American Chemical Society, 134(29), 11833-11835 (2012-07-04)
We report herein the first examples of asymmetric Mizoroki-Heck reactions using benzyl electrophiles. A new phosphoramidite was identified to be an effective chiral ligand in the palladium-catalyzed reaction. The reaction is compatible with polar functional groups and can be readily
Xiaoxiang Liu et al.
Journal of the American Chemical Society, 126(16), 5182-5191 (2004-04-22)
A general procedure for the palladium-catalyzed arylation of trimethylsilyl enolates of esters and imides is reported. In the presence of ZnF2 or Zn(O-t-Bu)2 as an additive, the trimethylsilyl enolates of esters, including those bearing alpha-alkoxy derivatives, underwent arylation in high
Bis (dibenzylideneacetone) palladium (0).
Stille JR, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)