675938
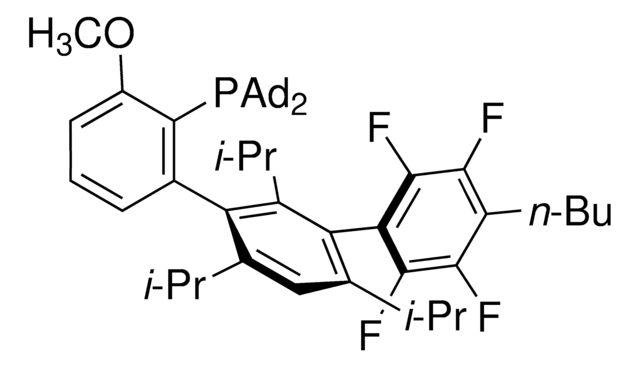
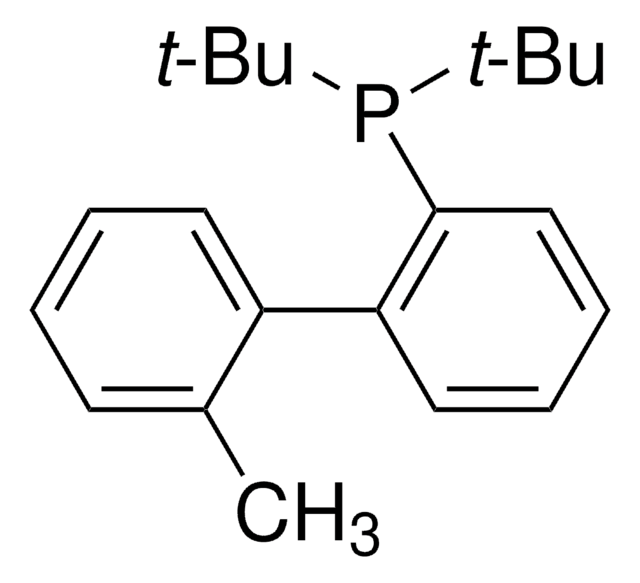
Me4tButylXphos
96%
동의어(들):
2-Di-tert-butylphosphino-3,4,5,6-tetramethyl-2′,4′,6′-triisopropyl-1,1′-biphenyl, Tetramethyl di-tBuXPhos, Tetramethyl tBuXPhos
About This Item
추천 제품
Quality Level
분석
96%
형태
solid
반응 적합성
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
mp
168-172 °C
작용기
phosphine
SMILES string
CC1=C(C)C(C)=C(C)C(C(C(C(C)C)=CC(C(C)C)=C2)=C2C(C)C)=C1P(C(C)(C)C)C(C)(C)C
InChI
1S/C33H53P/c1-19(2)26-17-27(20(3)4)30(28(18-26)21(5)6)29-24(9)22(7)23(8)25(10)31(29)34(32(11,12)13)33(14,15)16/h17-21H,1-16H3
InChI key
RCRYEYMHBHPZQD-UHFFFAOYSA-N
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
문서
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
관련 콘텐츠
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.