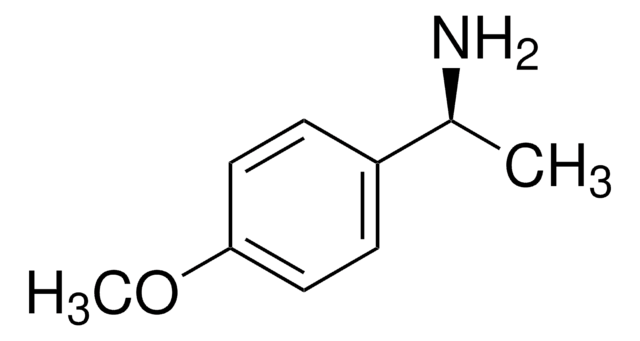
추천 제품
Grade
produced by BASF
분석
≥98.5% (GC)
99%
형태
liquid
광학 순도
enantiomeric excess: ≥98.5%
density
1.024 g/mL at 20 °C (lit.)
SMILES string
COc1ccc(cc1)[C@@H](C)N
InChI
1S/C9H13NO/c1-7(10)8-3-5-9(11-2)6-4-8/h3-7H,10H2,1-2H3/t7-/m1/s1
InChI key
JTDGKQNNPKXKII-SSDOTTSWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
(R)-(+)-4-Methoxy-α-methylbenzylamine can be used as a reactant to prepare:
- Enantiopure stereoisomers of hemicryptophanes, which are used for the recognition of glucopyranosides.
- Bicyclic Geissman-Waiss lactone via intramolecular ring-closure reaction of the diastereomeric mixture of sulfonium salts.
- N-[(1R)-1-(4-Methoxyphenyl)ethyl]-N′-methylthiourea by reacting with methyl isothiocyanate.
법적 정보
ChiPros is a registered trademark of BASF SE
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Improved hemicryptophane hosts for the stereoselective recognition of glucopyranosides
Schmitt A, et al.
Organic & Biomolecular Chemistry, 12(24), 4211-4217 (2014)
Synthesis of (+)-and (-)-Geissman-Waiss lactone from chiral sulfonium salts
Lopez-Gonzalez R, et al.
Tetrahedron Letters, 12(24), 151697-151697 (2020)
Yohan Park et al.
Archives of pharmacal research, 35(8), 1393-1401 (2012-09-04)
Thirty two thiourea derivatives were prepared and their agonistic activities on the retinoic acid receptor-related orphan receptor α (RORα) were evaluated. The replacement of the 3-allyl-2-imino-thiazolidin-4-one moiety of the lead compound CGP52608 (1) with various functional group substituted aromatic rings
문서
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.