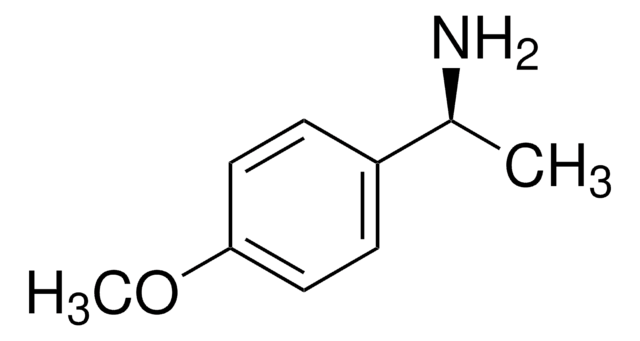
추천 제품
Grade
produced by BASF
Quality Level
분석
≥98.5% (GC)
99%
양식
liquid
광학 순도
enantiomeric excess: ≥98.5%
density
1.024 g/mL at 20 °C (lit.)
작용기
amine
SMILES string
COc1ccc(cc1)[C@H](C)N
InChI
1S/C9H13NO/c1-7(10)8-3-5-9(11-2)6-4-8/h3-7H,10H2,1-2H3/t7-/m0/s1
InChI key
JTDGKQNNPKXKII-ZETCQYMHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
(S)-(−)-4-Methoxy-α-methylbenzylamine is employed in the synthesis of S(+)-4-(1-phenylethylamino)quinazolines, as human immunoglobuline E inhibitor and haloaryl-β-amino acids. It is also used as a precursor to prepare chiral intermediate in the total synthesis of solanoeclepin A.
법적 정보
ChiPros is a registered trademark of BASF SE
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
S (+)-4-(1-phenylethylamino) quinazolines as inhibitors of human immunoglobuline E synthesis: potency is dictated by stereochemistry and atomic point charges at N-1.
Berger M, et al.
Journal of Medicinal Chemistry, 44(18), 3031-3038 (2001)
The asymmetric synthesis of β-haloaryl-β-amino acid derivatives.
Bull S D, et al.
Synlett, 2000(09), 1257-1260 (2000)
Novel Synthesis of the ABC Rings of Solanoeclepin A.
Lin Y T, et al.
Organic Letters, 16(22), 5948-5951 (2014)
문서
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.