추천 제품
Grade
produced by BASF
Quality Level
vapor pressure
0.5 mmHg ( 20 °C)
분석
≥99.0% (GC)
≥99.0%
형태
liquid
광학 순도
enantiomeric excess: ≥98.0%
refractive index
n20/D 1.526 (lit.)
bp
187-189 °C (lit.)
density
0.952 g/mL at 20 °C (lit.)
작용기
amine
phenyl
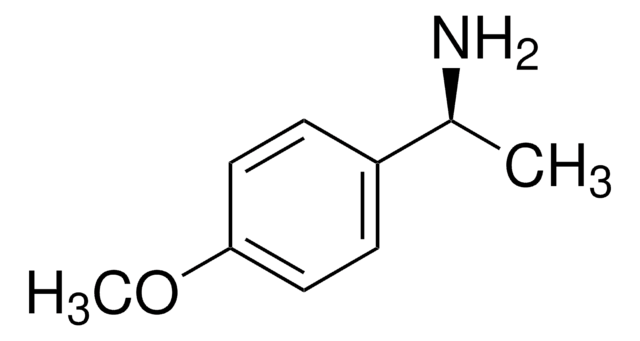
SMILES string
C[C@@H](N)c1ccccc1
InChI
1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3/t7-/m1/s1
InChI key
RQEUFEKYXDPUSK-SSDOTTSWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
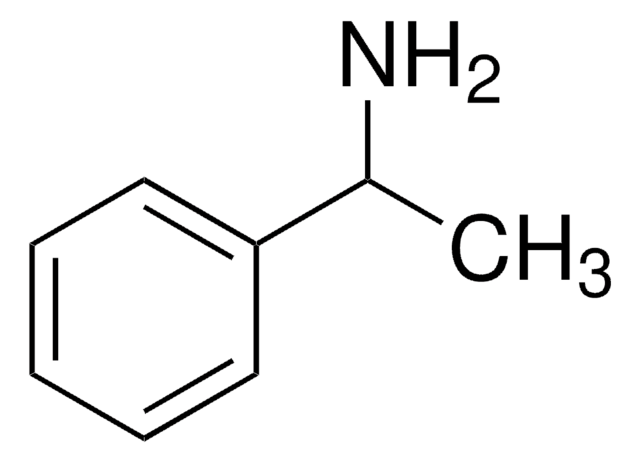
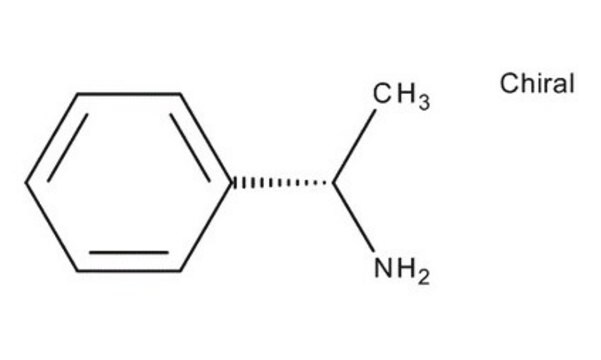
(R)-(+)-α-Methylbenzylamine is a chiral amine.
애플리케이션
(R)-(+)-α-Methylbenzylamine may be used as a substrate to synthesize:
- (S)-α-amino phosphonates
- N-{(S)-[cyclohexan-(S)-2-ol]}-(R)-α-methylbenzyl amine and N-{(R)-[cyclohexan-(R)-2-ol]}-(R)-α-methylbenzyl amine
- R-α-aminonitriles
법적 정보
ChiPros is a registered trademark of BASF SE
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point (°F)
158.0 °F - closed cup
Flash Point (°C)
70 °C - closed cup
이미 열람한 고객
Lithium perchlorate/diethylether catalyzed aminophosphonation of aldehydes.
Heydari A, et al.
Tetrahedron Letters, 39(37), 6729-6732 (1998)
New enantiomerically pure aminoalcohols from (R)-a-methylbenzylamine and cyclohexene oxide.
Barbaro P, et al.
Tetrahedron Asymmetry, 7(3), 843-850 (1996)
ChiPros Chiral Amines
Aldrich Chemfiles, 11(1) null
Karim Engelmark Cassimjee et al.
Organic & biomolecular chemistry, 10(28), 5466-5470 (2012-06-13)
For biocatalytic production of pharmaceutically important chiral amines the ω-transaminase enzymes have proven useful. Engineering of these enzymes has to some extent been accomplished by rational design, but mostly by directed evolution. By use of a homology model a key
Bartosz Lewandowski et al.
Chemical communications (Cambridge, England), (47)(47), 6399-6401 (2008-12-03)
Simple chiral aza-crown ethers based on sucrose display high enantioselectivity in complexation of phenylethylammonium chlorides.
문서
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.