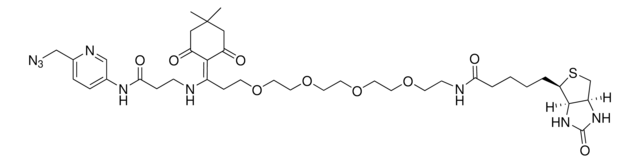
추천 제품
Quality Level
분석
95%
양식
solid
반응 적합성
reaction type: click chemistry
mp
55-64 °C
저장 온도
−20°C
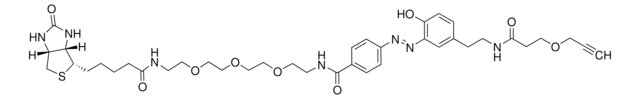
SMILES string
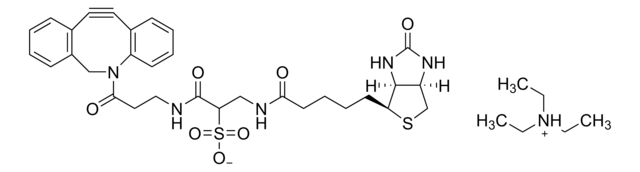
O=C(NCCOCCOCCOCCOCC#C)CCCC[C@@H](SC1)[C@@]2([H])[C@]1([H])NC(N2)=O
InChI
1S/C21H35N3O6S/c1-2-8-27-10-12-29-14-15-30-13-11-28-9-7-22-19(25)6-4-3-5-18-20-17(16-31-18)23-21(26)24-20/h1,17-18,20H,3-16H2,(H,22,25)(H2,23,24,26)/t17-,18-,20-/m1/s1
InChI key
SKMJWNZZFUDLKQ-QWFCFKBJSA-N
애플리케이션
Biotin-PEG4-alkyne may be used for the modification of 4-azidophenylalanine (AzPhe) silk fibroin via bioorthogonal azide–alkyne cycloaddition reaction for developing photopatternable protein material.
Biotinylation reagent for labeling azide containing molecules or biomolecules using copper-catalyzed 1,3 dipolar cycloaddition click chemistry. The alkyne group reacts with azides to form a stable triazole linkage, facilitating the introduction of biotin into your azide modified system of interest.
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
기타 정보
Chemoproteomic Profiling of Gut Microbiota-Associated Bile Salt Hydrolase Activity
Fluorescent Heterotelechelic Single-Chain Polymer Nanoparticles: Synthesis, Spectroscopy, and Cellular Imaging
Arginine-Selective Chemical Labeling Approach for Identification and Enrichment of Reactive Arginine Residues in Proteins
Selective Imaging of Gram-Negative and Gram-Positive Microbiotas in the Mouse Gut
Metabolic Oligosaccharide Engineering with Alkyne Sialic Acids Confers Neuraminidase Resistance and Inhibits Influenza Reproduction
A Modular Probe Strategy for Drug Localization, Target Identification and Target Occupancy Measurement on Single Cell Level
Fluorescent Heterotelechelic Single-Chain Polymer Nanoparticles: Synthesis, Spectroscopy, and Cellular Imaging
Arginine-Selective Chemical Labeling Approach for Identification and Enrichment of Reactive Arginine Residues in Proteins
Selective Imaging of Gram-Negative and Gram-Positive Microbiotas in the Mouse Gut
Metabolic Oligosaccharide Engineering with Alkyne Sialic Acids Confers Neuraminidase Resistance and Inhibits Influenza Reproduction
A Modular Probe Strategy for Drug Localization, Target Identification and Target Occupancy Measurement on Single Cell Level
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Lili Lu et al.
Angewandte Chemie (International ed. in English), 54(33), 9679-9682 (2015-06-24)
Glycosylphosphatidylinositol (GPI) anchoring of proteins to the cell surface is important for various biological processes, but GPI-anchored proteins are difficult to study. An effective strategy was developed for the metabolic engineering of cell-surface GPIs and GPI-anchored proteins by using inositol
Nrf1 can be processed and activated in a proteasome-independent manner.
Vangala JR, et al.
Current Biology, 26(18), R834-R835 (2016)
Maheshika S K Wanigasekara et al.
ACS omega, 3(10), 14229-14235 (2019-08-29)
Modification of arginine residues using dicarbonyl compounds is a common method to identify functional or reactive arginine residues in proteins. Arginine undergoes several kinds of posttranslational modifications in these functional residues. Identifying these reactive residues confidently in a protein or
Masanobu Nagano et al.
Bioorganic & medicinal chemistry, 25(21), 5952-5961 (2017-10-11)
Vaccination is a reliable method of prophylaxis and a crucial measure for public health. However, the majority of vaccines cannot be administered orally due to their degradation in the harsh gut environment or inability to cross the GI tract. In
Anna Rutkowska et al.
ACS chemical biology, 11(9), 2541-2550 (2016-07-08)
Late stage failures of candidate drug molecules are frequently caused by off-target effects or inefficient target engagement in vivo. In order to address these fundamental challenges in drug discovery, we developed a modular probe strategy based on bioorthogonal chemistry that
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.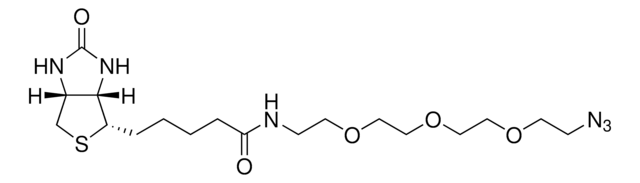
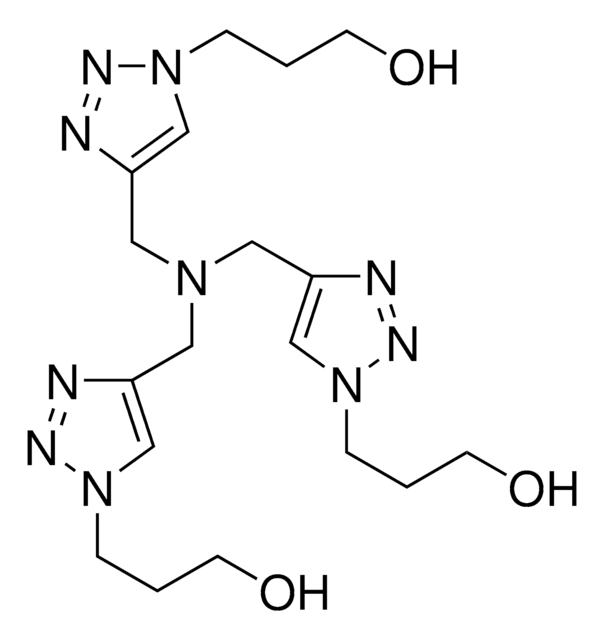
![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)