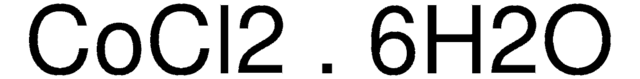추천 제품
Grade
for analytical purposes
Quality Level
vapor pressure
40 mmHg ( 0 °C)
분석
≥97%
97.0-102.0% (KT)
양식
solid
음이온 미량물
nitrate (NO3-): ≤0.01%
sulfate (SO42-): ≤0.007%
양이온 미량물
Fe: ≤0.005%
Ni: ≤0.15%
Pb: ≤0.002%
Zn: ≤0.05%
SMILES string
O.O.O.O.O.O.Cl[Co]Cl
InChI
1S/2ClH.Co.6H2O/h2*1H;;6*1H2/q;;+2;;;;;;/p-2
InChI key
GFHNAMRJFCEERV-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Cobalt(II) chloride hexahydrate, a hydrated form of cobalt chloride, is employed in electroplating and catalyst preparation. It serves as a precursor for synthesizing electrode materials for lithium-ion batteries and acts as a catalyst in a range of organic reactions, including acetylation, tosylation of alcohols, and condensation reactions.
애플리케이션
Cobalt(II) chloride hexahydrate can be used as:
- An additive to the electron transport layer (ETL) in perovskite solar cells to improve their performance, particularly by reducing energy losses and increasing the open-circuit voltage.
- A cobalt source for doping ZnO nanostructures. The incorporation of cobalt ions into the ZnO matrix is crucial for modifying its electronic and optical properties.
- A precursor to modify cobalt metal-organic framework (Co-MOF) derived carbon microspheres for application as anode materials in lithium-ion batteries.
분석 메모
Substances not precipitated by ammonium sulfide (as sulphates) ≤ 0.3 %
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Hyeohn Kim et al.
ACS nano, 15(1), 979-988 (2020-12-18)
Chiral inorganic nanomaterials have revealed opportunities in various fields owing to their strong light-matter interactions. In particular, chiral metal oxide nanomaterials that can control light and biochemical reactions have been highlighted due to their catalytic activity and biocompatibility. In this
Electroplating and characterization of cobalt-nickel-iron and nickel-iron for magnetic microsystems applications
Rasmussen, FE., et al.
Sensors and actuators A, Physical, 92, 242-248 (2001)
One-step synthesis of cobalt and nitrogen co-doped carbon nanotubes and their catalytic activity for the oxygen reduction reaction
Fu, S., et al.
Journal of Material Chemistry A, 3, 12718-12722 (2015)
Macharla Arun Kumar et al.
Journal of hazardous materials, 340, 399-406 (2017-07-25)
We developed a novel stabilized nanoscale zerovalent iron (NZVI) particles with Ni using an electron conducting polymer, polyvinylpyrrolidone (PVP), to selectively dechlorinate trichloroethylene (TCE) to non-toxic intermediates. The size of the PVP stabilized NZVI-Ni ((PVP-NZVI-Ni), average diameter: ∼20nm) is smaller
Sebastian Klemenz et al.
ChemSusChem, 11(18), 3150-3156 (2018-07-27)
High-performance catalysts for the oxygen-evolution reaction in water electrolysis are usually based on expensive and rare elements. Herein, mixed-metal borides are shown to be competitive with established electrocatalysts like noble metal oxides and other transition-metal(oxide)-based catalysts. Iron incorporation into nanoscale
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.