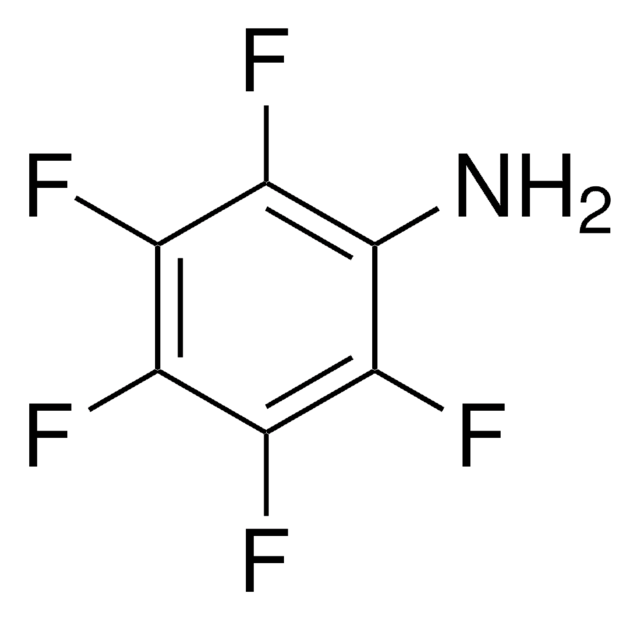
추천 제품
Quality Level
분석
97%
형태
liquid
반응 적합성
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
refractive index
n20/D 1.431 (lit.)
n20/D 1.432
bp
186 °C (lit.)
density
1.662 g/mL at 25 °C
1.687 g/mL at 25 °C (lit.)
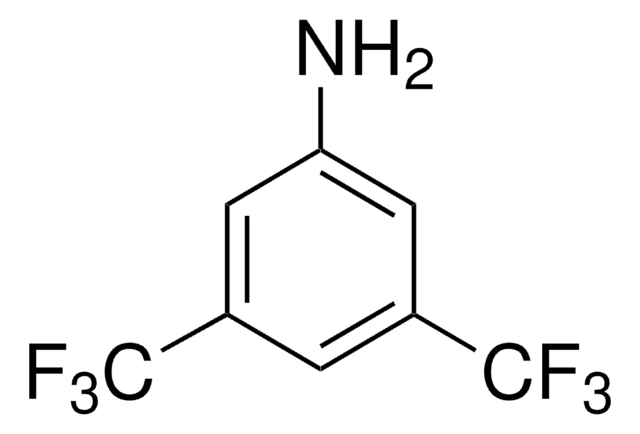
작용기
fluoro
SMILES string
Nc1c(F)c(F)c(c(F)c1F)C(F)(F)F
InChI
1S/C7H2F7N/c8-2-1(7(12,13)14)3(9)5(11)6(15)4(2)10/h15H2
InChI key
FJOACTZFMHZHSC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
기타 정보
Acidic amides are superior directing groups for promoting C-H activation reactions with both Pd(0)/PR3 and Pd(II) catalysts.
Used in the Preparation of
Used in the Preparation of
- Lactams via palladium-catalyzed olefination of arylamides with benzylacrylate, followed by 1,4-conjugate addition
- N-(fluorinated aryl)benzamides as substrates for regioselective C-H amination reactions with O-benzoylhydroxylamines
- Substituted succinimides via palladium-catalyzed carbonylation of N-aryl amides
- N-aryl cyclopropanecarboxamide substrates and various amino acid ligands for palladium-catalyzed C-H activation of cyclopropanes
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Eun Jeong Yoo et al.
Journal of the American Chemical Society, 132(49), 17378-17380 (2010-11-19)
Pd(II)-catalyzed β-C(sp(3))-H carbonylation of N-arylamides under CO (1 atm) has been achieved. Following amide-directed C(sp(3))-H cleavage and insertion of CO into the resulting [Pd(II)-C(sp(3))] bond, intramolecular C-N reductive elimination gave the corresponding succinimides, which could be readily converted to 1,4-dicarbonyl
Eun Jeong Yoo et al.
Journal of the American Chemical Society, 133(20), 7652-7655 (2011-04-28)
C-H amination of N-aryl benzamides with O-benzoyl hydroxylamines has been achieved with either Pd(II) or Pd(0) catalysts. Furthermore, we demonstrate that secondary amines can be directly used with benzoyl peroxide in a one-pot procedure that proceeds via the in situ
Wasa, M;
Journal of the American Chemical Society, 132(11), 3680-3681 null
Palladium(0)-Catalyzed Alkynylation of C(sp3)-H Bonds
He, J.:
Journal of the American Chemical Society null
Masayuki Wasa et al.
Journal of the American Chemical Society, 133(49), 19598-19601 (2011-11-09)
Systematic ligand development has led to the identification of novel mono-N-protected amino acid ligands for Pd(II)-catalyzed enantioselective C-H activation of cyclopropanes. A diverse range of organoboron reagents can be used as coupling partners, and the reaction proceeds under mild conditions.
관련 콘텐츠
Yu program focuses on efficient C–H bond activation for drug synthesis, using simple starting materials.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.