모든 사진(1)
About This Item
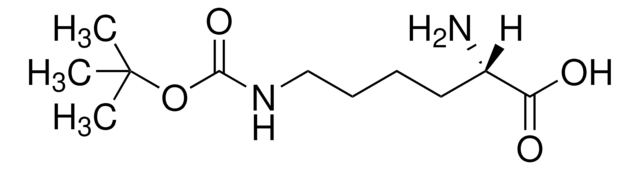
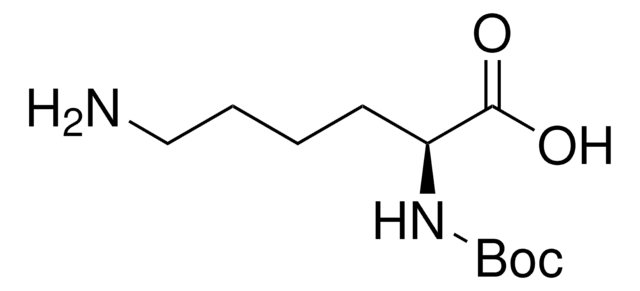
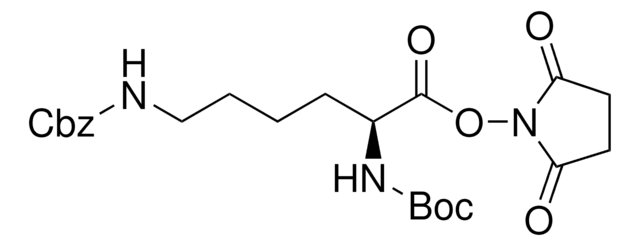
Linear Formula:
NH2(CH2)4CH(COOH)NHCOOC(CH3)3
CAS Number:
Molecular Weight:
246.30
Beilstein:
4252546
MDL number:
UNSPSC 코드:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
형태
solid
광학 활성
[α]20/D +22°, c = 2 in methanol
반응 적합성
reaction type: Boc solid-phase peptide synthesis
mp
~205 °C (dec.) (lit.)
응용 분야
peptide synthesis
SMILES string
CC(C)(C)OC(=O)N[C@@H](CCCCN)C(O)=O
InChI
1S/C11H22N2O4/c1-11(2,3)17-10(16)13-8(9(14)15)6-4-5-7-12/h8H,4-7,12H2,1-3H3,(H,13,16)(H,14,15)/t8-/m0/s1
InChI key
DQUHYEDEGRNAFO-QMMMGPOBSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Boc-Lys-OH (Nα-Boc-L-lysine) can be used as a building block to synthesize:
- A heterotrifunctional peptide-based linker molecule applicable as a bio-labeling reagent.
- Lysine derivatives of azamacrocycle and anthraquinone.
- Boc-Lys(Bn4-DTPA)-OH, a precursor to synthesize diethylene triamine pentaacetic acid (DTPA) containing peptides.
- A ferrocene-amino acid conjugate which is used in developing chemical warfare agent (CWA) sensor.
교체됨
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Synthesis of Nα-Boc-Nε-tetrabenzyl-DTPA-l-lysine and Nα-Fmoc-Nε-tetra-t-butyl-DTPA-l-lysine, building blocks for solid phase synthesis of DTPA-containing peptides
Davies JS and Al-Jamri L
Journal of Peptide Science, 8(12), 663-670 (2002)
Synthesis of lysine derivatives containing aza-crown ethers and a chromophore unit
Ossowski T, et al.
Tetrahedron Letters, 46(10), 1735-1738 (2005)
Carla Kühn et al.
Journal of agricultural and food chemistry, 66(26), 6727-6733 (2018-06-09)
Glucosinolates and their breakdown products, especially isothiocyanates (ITCs), are hypothesized to exert a broad range of bioactivities. However, physiological mechanisms are not yet completely understood. In this study, formation of protein conjugates after incubation with benzyl isothiocyanate (BITC) was investigated
A novel heterotrifunctional peptide-based cross-linking reagent for facile access to bioconjugates. Applications to peptide fluorescent labelling and immobilisation
Clave G, et al.
Organic & Biomolecular Chemistry, 6(17), 3065-3078 (2008)
Carla Kühn et al.
Molecular nutrition & food research, 62(20), e1800588-e1800588 (2018-08-10)
Different metabolic and excretion pathways of the benzyl glucosinolate breakdown products benzyl isothiocyanate and benzyl cyanide are investigated to obtain information about their multiple fate after ingestion. Detailed focus is on the so far underestimated transformation/excretion pathways-protein conjugation and exhalation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.