806501
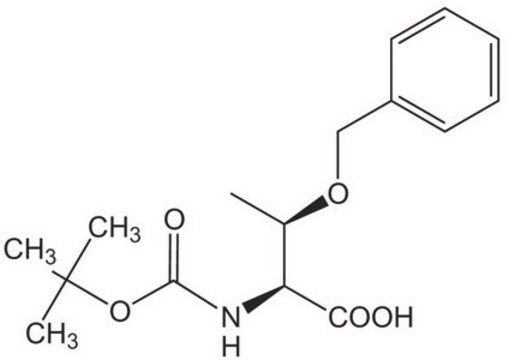
Boc-Thr(t-Bu)-OH
동의어(들):
(S)-3-tert-Butoxy-2-tert-butoxycarbonylamino-butyric acid, Boc-O-tert-butyl-L-threonine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
(CH3)3COCH(CH3)CH(COOH)NHCOOC(CH3)3
CAS Number:
Molecular Weight:
275.34
Beilstein:
4454820
MDL number:
UNSPSC 코드:
12352209
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
95%
Quality Level
양식
powder
mp
98.9 °C
응용 분야
peptide synthesis
저장 온도
2-8°C
SMILES string
C[C@@H](OC(C)(C)C)[C@H](NC(=O)OC(C)(C)C)C(O)=O
InChI
1S/C13H25NO5/c1-8(18-12(2,3)4)9(10(15)16)14-11(17)19-13(5,6)7/h8-9H,1-7H3,(H,14,17)(H,15,16)/t8-,9+/m1/s1
InChI key
LKRXXARJBFBMCE-BDAKNGLRSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Boc-Thr(t-Bu)-OH (N-Boc-O-tert-butyl-L-threonine) participates in the synthesis of 2,3-unsaturated glycosides, via reaction with per-O-acetylated glucal in the presence of Er(OTf)3 catalyst.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Antonio Procopio et al.
Carbohydrate research, 342(14), 2125-2131 (2007-06-23)
Er(OTf)(3) is a useful catalyst for the Ferrier rearrangement furnishing high yields of O- and S-glycosides. The transformation has wide applicability, cleaner reaction profiles, mild reaction conditions, and high stereoselectivity and the catalyst, which is also commercially available, can be
Christian Haenig et al.
Cell reports, 32(7), 108050-108050 (2020-08-20)
Interactome maps are valuable resources to elucidate protein function and disease mechanisms. Here, we report on an interactome map that focuses on neurodegenerative disease (ND), connects ∼5,000 human proteins via ∼30,000 candidate interactions and is generated by systematic yeast two-hybrid
관련 콘텐츠
Yu program focuses on efficient C–H bond activation for drug synthesis, using simple starting materials.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.