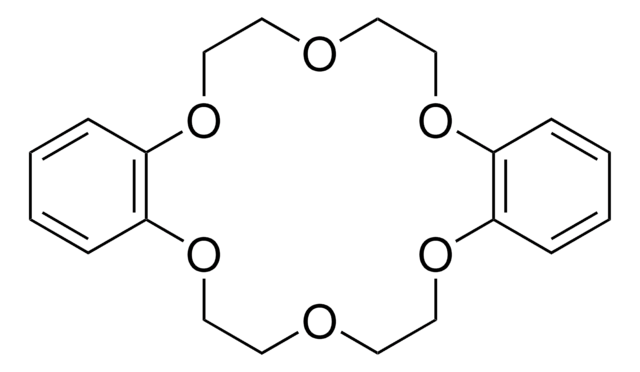
추천 제품
형태
liquid
농도
1.0 M in THF
density
0.944 g/mL
InChI
1S/C12H24O6/c1-2-14-5-6-16-9-10-18-12-11-17-8-7-15-4-3-13-1/h1-12H2
InChI key
XEZNGIUYQVAUSS-UHFFFAOYSA-N
관련 카테고리
애플리케이션
18-Crown-6 may be used to catalyze the N-alkylation of heterocyclic compounds and allylation of functionalized aldehydes.
Additive for greener etherification using KF-alumina.
Additive for greener etherification using KF-alumina.
Synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride-alumina and 18-crown-6
Synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride-alumina and 18-crown-6
Phase-transfer catalyst used in a chemoselective reduction of fused tetrazoles with NaBH4 and potassium hydroxide.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
-2.0 °F
Flash Point (°C)
-18.88 °C
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Synthesis, 3275-3275 (2006)
Phase-transfer alkylation of heterocycles in the presence of 18-crown-6 and potassium tert-butoxide.
Guida WC and Mathre DJ
The Journal of Organic Chemistry, 45(16), 3172-3176 (1980)
Preparation and purification of 18-crown-6 [1, 4, 7, 10, 13, 16-hexaoxacyclooctadecane].
Gokel GW, et al.
The Journal of Organic Chemistry, 39(16), 2445-2446 (1974)
Sergey A Dergunov et al.
Journal of the American Chemical Society, 133(49), 19656-19659 (2011-11-15)
We describe a new co-entrapment and release motif based on the combination of noncovalent and steric interactions in materials with well-defined nanopores. Individual components enter hollow nanocapsules through nanopores in the capsule shell. Their complex, larger than the pore size
Hiroaki Kotani et al.
Journal of the American Chemical Society, 133(29), 11092-11095 (2011-06-28)
Addition of potassium superoxide with 18-crown-6 ether (KO(2)(•-)-18-crown-6) to a toluene solution of an acridinium ion-linked porphyrin triad (Acr(+)-H(2)P-Acr(+)) resulted in a remarkable enhancement of the fluorescence intensity. Thus, Acr(+)-H(2)P-Acr(+) acts as an efficient fluorescence sensor for superoxide. Electron transfer
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.