모든 사진(2)
About This Item
실험식(Hill 표기법):
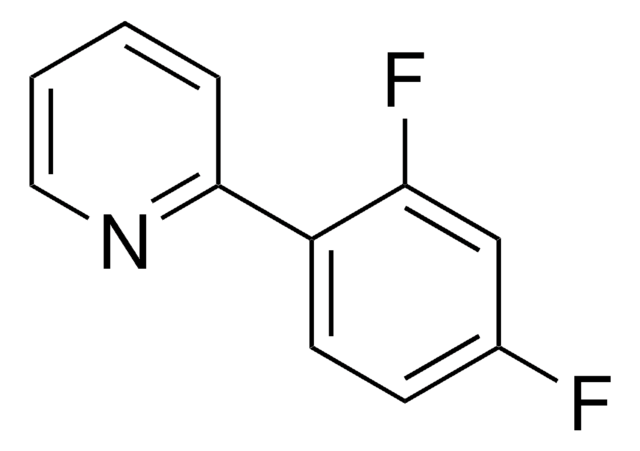
C12H6F5N
CAS Number:
Molecular Weight:
259.17
MDL number:
UNSPSC 코드:
12352101
NACRES:
NA.22
추천 제품
분석
≥95%
형태
powder or crystals
반응 적합성
reaction type: Photocatalysis
reagent type: catalyst
mp
59-64 °C
InChI
1S/C12H6F5N/c13-8-2-3-9(10(14)5-8)11-4-1-7(6-18-11)12(15,16)17/h1-6H
InChI key
FMKQPMDFNYNYAG-UHFFFAOYSA-N
애플리케이션
2-(2,4-Difluorophenyl)-5-(trifluoromethyl)pyridine is a ligand used for the preparation of Ir(III) photocatalysts.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Kazimer L Skubi et al.
Journal of the American Chemical Society, 139(47), 17186-17192 (2017-11-01)
Stereochemical control of electronically excited states is a long-standing challenge in photochemical synthesis, and few catalytic systems that produce high enantioselectivities in triplet-state photoreactions are known. We report herein an exceptionally effective chiral photocatalyst that recruits prochiral quinolones using a
Thomas Rossolini et al.
Organic letters, 20(21), 6794-6798 (2018-10-24)
A visible-light-mediated photocatalytic umpolung synthesis of 1,3-diamines from in situ-generated imines and dehydroalanine derivatives is described. Pivoting on a key nucleophilic addition of photocatalytically generated α-amino radicals to electron-deficient alkenes, this three-component coupling reaction affords 1,3-diamines efficiently and diastereoselectively. The
Timothy M Monos et al.
Science (New York, N.Y.), 361(6409), 1369-1373 (2018-09-29)
Alkene aminoarylation with a single, bifunctional reagent is a concise synthetic strategy. We report a catalytic protocol for the addition of arylsulfonylacetamides across electron-rich alkenes with complete anti-Markovnikov regioselectivity and excellent diastereoselectivity to provide 2,2-diarylethylamines. In this process, single-electron alkene
John C Tellis et al.
Science (New York, N.Y.), 345(6195), 433-436 (2014-06-07)
The routine application of C(sp3)-hybridized nucleophiles in cross-coupling reactions remains an unsolved challenge in organic chemistry. The sluggish transmetalation rates observed for the preferred organoboron reagents in such transformations are a consequence of the two-electron mechanism underlying the standard catalytic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)

