추천 제품
Quality Level
분석
≥95%
양식
powder
저장 온도
−20°C
SMILES string
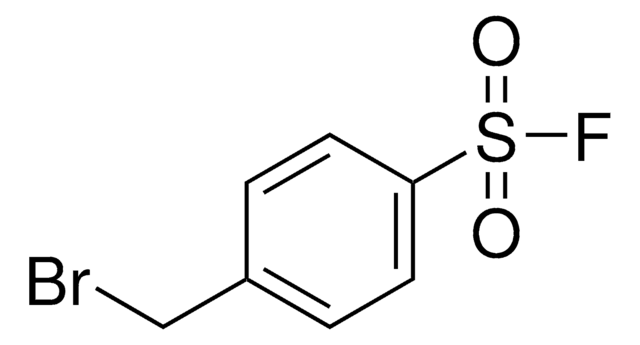
F[S](=O)(=O)c1ccc(cc1)CCNC(=O)CCCC#C
InChI
1S/C14H16FNO3S/c1-2-3-4-5-14(17)16-11-10-12-6-8-13(9-7-12)20(15,18)19/h1,6-9H,3-5,10-11H2,(H,16,17)
애플리케이션
4-(2-(Hex-5-ynamido)ethyl)benzenesulfonyl fluoride is an alkyne-functionalized sulfonyl fluoride activity-based probe (SFABP). Sulfonyl fluorides covalently modify reactive serines in addition to threonine, lysine, tyrosine, cysteine, and histidine residues in a context-specific manner. Activity-based probes are useful for the targeting, isolation, and identification of proteins. Furtermore, the alkyne handle on SFABP enables click chemistry-based incorpoation of azide tags, such as fluorophores or biotins, for visualization or enrichment and detection, respectively.
기타 정보
Unbiased Mass Spectrometry Elucidation of the Targets and Mechanisms of Activity-Based Probes: A Case Study Involving Sulfonyl Fluorides
Sulfonyl fluorides as privileged warheads in chemical biology
Chemical Proteomics with Sulfonyl Fluoride Probes Reveals Selective Labeling of Functional Tyrosines in Glutathione Transferase
Sulfonyl Fluoride Analogues as Activity-Based Probes for Serine Proteases
Europium-Labeled Activity-Based Probe through Click Chemistry: Absolute Serine Protease Quantification Using 153Eu Isotope Dilution ICP/MS
Sulfonyl fluorides as privileged warheads in chemical biology
Chemical Proteomics with Sulfonyl Fluoride Probes Reveals Selective Labeling of Functional Tyrosines in Glutathione Transferase
Sulfonyl Fluoride Analogues as Activity-Based Probes for Serine Proteases
Europium-Labeled Activity-Based Probe through Click Chemistry: Absolute Serine Protease Quantification Using 153Eu Isotope Dilution ICP/MS
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
가장 최신 버전 중 하나를 선택하세요:
Xiaowen Yan et al.
Angewandte Chemie (International ed. in English), 51(14), 3358-3363 (2012-02-22)
Click and analyze: the titled probe was synthesized by conjugating a sulfonyl fluoride and azido unit using click chemistry to give SF-Eu, which can react specifically with serine (Ser) in the active site of serine protease (SP). Combination of the
Arjun Narayanan et al.
Chemical science, 6(5), 2650-2659 (2015-05-01)
Sulfonyl fluoride electrophiles have found significant utility as reactive probes in chemical biology and molecular pharmacology. As warheads they possess the right balance of biocompatibility (including aqueous stability) and protein reactivity. Their functionality is privileged in this regard as they
Christian Gu et al.
Chemistry & biology, 20(4), 541-548 (2013-04-23)
Chemical probes have great potential for identifying functional residues in proteins in crude proteomes. Here we studied labeling sites of chemical probes based on sulfonyl fluorides (SFs) on plant and animal proteomes. Besides serine proteases and many other proteins, SF-based
D Alexander Shannon et al.
Chembiochem : a European journal of chemical biology, 13(16), 2327-2330 (2012-09-26)
Enriched with fluoride: To expand on the available tools to interrogate proteases, we explored sulfonyl fluorides as activity-based probes. An alkyne-tagged sulfonyl fluoride covalently modifies members of the S1 family of serine proteases. By applying click chemistry, avidin enrichment and
Thomas E J Chavas et al.
ACS chemical biology, 13(10), 2897-2907 (2018-09-08)
The elucidation of protein/drug interactions remains a major challenge in drug discovery. Liquid chromatography-tandem mass spectrometry has emerged as a tremendously powerful technology for this endeavor, but its full potential has yet to be realized owing in part to unresolved
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.