추천 제품
양식
liquid
반응 적합성
reaction type: click chemistry
SMILES string
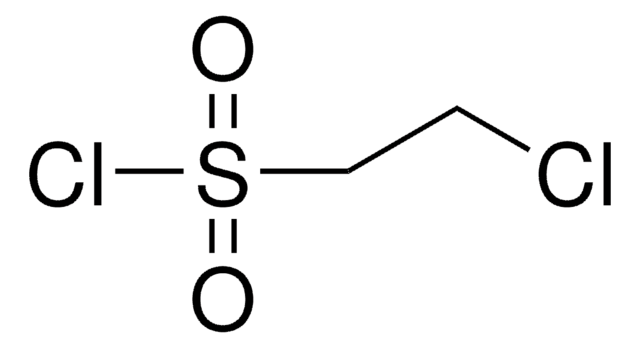
F[S](=O)(=O)C(Br)CBr
InChI key
NIJDNCLEFCJVRL-UHFFFAOYSA-N
애플리케이션
1,2-Dibromoethane-1-sulfonyl fluoride (DESF) is a bench-stable precursor to 1-bromoethene-1-sulfonyl fluoride (BESF), a new and robust connective hub for the Sulfur (VI) fluoride exchange (SuFEx) click reaction. BESF offers similar routes as ethenesulfonyl fluoride (ESF, cat# 746959) but with additional reactivity due to the embedded bromo group.
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Treatment of DESF with triethylamine generates BESF in situ, which has been used to synthesize diverse and unprecented sulfonyl fluorides in good-to-excellent yields. As the resulting molecules possess sulfonyl fluoride handles, further SuFEx reactions are also possible. DESF adds another useful tool for conjugation strategies in chemical biology and organic and polymer synthesis.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Jing Leng et al.
Chemical communications (Cambridge, England), 54(35), 4477-4480 (2018-04-17)
A new fluorosulfonylation reagent 1-bromoethene-1-sulfonyl fluoride was developed (1-Br-ESF). This unique reagent possesses three addressable handles (vinyl, bromide, and sulfonyl fluoride) and has great potential to function as a tris-electrophile and as a sulfur(vi) fluoride exchange (SuFEx) clickable material to
Christopher J Smedley et al.
Chemical communications (Cambridge, England), 54(47), 6020-6023 (2018-05-26)
We demonstrate 1,2-dibromoethane-1-sulfonyl fluoride (DESF) as a bench-stable and readily accessible precursor to the robust SuFEx connector, 1-bromoethene-1-sulfonyl fluoride (BESF). The in situ generation of BESF from DESF opens up several new reaction profiles, including application in the syntheses of
Joice Thomas et al.
Organic letters, 20(13), 3749-3752 (2018-06-16)
A regioselective metal-free preparation of 4-fluorosulfonyl 1,2,3-triazoles from organic azides and a hitherto underexplored bromovinylsulfonyl fluoride building block is described. This reaction is very general and was extended to the synthesis of various sulfonates, sulfonamides, and sulfonic acid derivatives of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.